Key Points
HGBL-DH/TH makes up 8% of de novo DLBCL, with HGBL-DH/TH with BCL2 rearrangement being a GCB phenomenon.
Restricting FISH testing to tumors with dual protein expression and GCB subtype results in testing <15% of tumors, but missing ∼35% of HGBL-DH/TH.
Abstract
High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (HGBL-DH/TH) is a newly defined entity in the latest World Health Organization Classification. Accurate diagnosis would appear to mandate fluorescence in situ hybridization (FISH) for all tumors with diffuse large B-cell lymphoma (DLBCL) morphology. We present the results of FISH, cell-of-origin, and immunohistochemistry (IHC) testing from 1228 DLBCL biopsies from 3 clinical trials and a population-based registry. HGBL-DH/TH made up 7.9% of the DLBCL, confined primarily to the germinal center B-cell–like (GCB; 13.3%) compared with activated B-cell-like (ABC; 1.7%) subtype (P < .001). HGBL-DH/TH with BCL2 rearrangement is a GCB phenomenon with no cases observed in 415 ABC DLBCL. A screening strategy restricting FISH testing to tumors of GCB subtype (by Lymph2Cx or Hans IHC) plus dual protein expression of MYC and BCL2 by IHC could limit testing to 11% to 14% of tumors, with a positive predictive value of 30% to 37%; however, this strategy would miss approximately one-quarter of tumors with HBGL-DH/TH with BCL2 rearrangement and one-third of all HGBL-DH/TH. These results provide accurate estimation of the proportion of HGBL-DH/TH among tumors with DLBCL morphology and allow determination of the impact of various methods available to screen DLBCL tumors for FISH testing.
Introduction
The World Health Organization 2017 Classification of Tumors of Hematopoietic and Lymphoid Tissues has introduced a new entity: high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (HGBL-DH/TH).1 The proportion of HGBL-DH/TH among tumors with diffuse large B-cell lymphoma (DLBCL) morphology is estimated to be 1% to 12%.2-6 Historically, fluorescence in situ hybridization (FISH) for MYC rearrangement on DLBCL tumors has been performed only in the presence of unusual morphological features, high proliferation rate, or at the request of the treating physician. However, it has been established that morphological features and proliferation rate cannot reliably identify HGBL-DH/TH tumors.1,7 Although identification of HGBL-DH/TH tumors with DLBCL morphology would appear to require that FISH be performed on every DLBCL, limited laboratory resources and, in some cases, limited biopsy tissue has led to recommendations regarding potential screening strategies to select tumors for FISH testing. Proposed strategies include performing FISH in tumors of germinal B-cell–like (GCB) cell-of-origin (COO) subtype and/or tumors that have dual protein expression (DPE) of MYC and BCL2 by immunohistochemistry (IHC).8 Here we present the results of performing FISH, COO testing, and IHC for MYC and BCL2 on a large cohort of tumors with DLBCL morphology drawn from a population-based registry and 3 clinical trials, allowing accurate estimation of the incidence of HGBL-DH/TH and the effects of screening strategies on detection of this entity.
Study design
Break-apart FISH testing (Vysis LSI, Abbott) was performed on biopsies with DLBCL morphology from population-based registry cohorts from the British Columbia Cancer Agency9 (tissue microarrays [TMAs] and whole sections), the Conventional Chemo Vs HD Chemo Followed by Auto SCT in Younger Pts With Aggressive Non-Hodgkin's Lymphoma trial (MegaCHOEP; TMAs)10 , Rituximab with CHOP Over Age 60 Years trial (RICOVER60; TMAs )11-14 , and US Intergroup E1412 (sections) trial. FISH testing was performed for MYC, BCL2, and BCL6 in all cases with the exception of the E1412 trial, in which BCL2 and BCL6 FISH was performed only when MYC rearrangement was detected. Scoring of IHC for MYC and BCL2 was performed by expert hematopathologists (A.M., P.F., G.W.S., R.D.G. [British Columbia Cancer Agency], G.O., A.R. [MegaCHOEP and RICOVER60], and R.L.K., W.R.M. [E1412]) using thresholds of ≥40% and ≥50% to define positivity, respectively.15,16 COO was assigned using the Lymph2Cx gene expression-based assay17 and the IHC-based Hans algorithm.15 See “Patient Cohorts” in the supplemental Data, available on the Blood Web site, for inclusion criteria for patient cohorts and details of laboratory testing.
Results and discussion
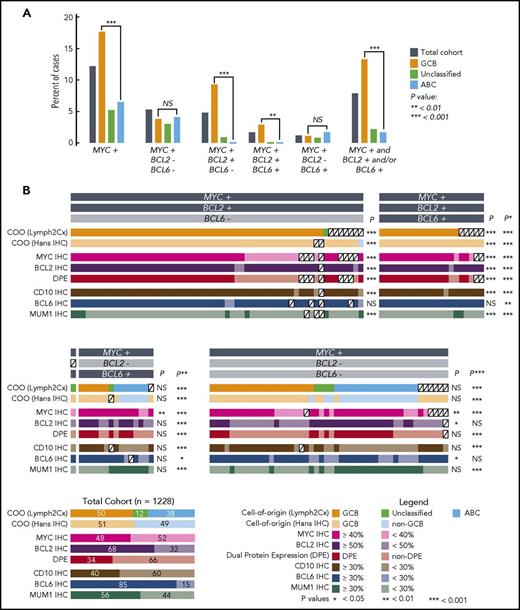
MYC rearrangement was detected in 12.2% of 1228 biopsies, with a significantly higher proportion of GCB (17.7%) than activated B-cell-like (ABC) subtype (6.5%) harboring rearrangements (P < .001, Fisher’s exact test, Figure 1A). MYC as the sole rearrangement (5.3%) and HGBL-DH with BCL6 rearrangement (1.2%) was observed in both COO subtypes. In contrast, HGBL-DH with BCL2 (4.8%) and HBGL-TH (1.7%) are GCB phenomena, with no cases detected in 415 ABC tumors tested (P < .001, Fisher’s exact test). In total, 7.9% of tumors with DLBCL morphology were assigned to the HBGL-DH/TH entity, comprising 13.3% of GCB and 1.7% of ABC DLBCL (P < .001, Fisher’s exact test). These results are consistent with estimates from previously reported smaller studies. Exceptions include the higher rate of MYC rearrangements compared with that reported by Copie-Bergman et al5 and the small proportion of HGBL-DH/TH with BCL2 rearrangement reported to be ABC/non-GCB in some studies.3,16,18 These discrepancies likely relate to use of a MYC break-apart probe set (DAKO) that fails to detect a proportion of chromosomal breaks telomeric to the MYC gene19 and use of COO tests with inferior accuracy, respectively.
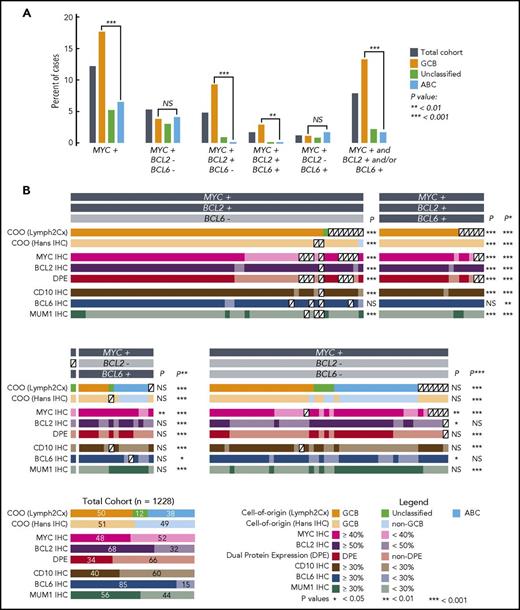
Characteristics of tumors with DLBCL morphology that have MYC rearrangement with or without BCL2 and/or BCL6 rearrangements. (A) Percentages of tumors that harbor MYC rearrangement in the presence or absence of concomitant BCL2 and/or BCL6 rearrangements. Analyses involving COO are based on the 1098 tumors in the cohort in which COO was determined using the Lymph2Cx gene expression-based assay. (B) COO and IHC results for tumors with MYC rearrangement. Each column represents an individual tumor, with the exception of the total cohort in the bottom left corner, included to show percentages of the characteristics in the 1228 patients. P values are for Fisher’s exact tests comparing the frequency of the characteristic in the group vs the total cohort. Additional comparisons were: *P: HGBL-DH/TH with BCL2 rearrangement group vs the total cohort; **P: all HGBL-DH/TH vs the total cohort; ***P: MYC rearrangement group vs the total cohort.
Characteristics of tumors with DLBCL morphology that have MYC rearrangement with or without BCL2 and/or BCL6 rearrangements. (A) Percentages of tumors that harbor MYC rearrangement in the presence or absence of concomitant BCL2 and/or BCL6 rearrangements. Analyses involving COO are based on the 1098 tumors in the cohort in which COO was determined using the Lymph2Cx gene expression-based assay. (B) COO and IHC results for tumors with MYC rearrangement. Each column represents an individual tumor, with the exception of the total cohort in the bottom left corner, included to show percentages of the characteristics in the 1228 patients. P values are for Fisher’s exact tests comparing the frequency of the characteristic in the group vs the total cohort. Additional comparisons were: *P: HGBL-DH/TH with BCL2 rearrangement group vs the total cohort; **P: all HGBL-DH/TH vs the total cohort; ***P: MYC rearrangement group vs the total cohort.
Optimally, a screening test would identify a population that encompasses most or all HGBL-DH/TH (ie, have high sensitivity) while significantly reducing the number of cases requiring FISH. Comparison of the incidence of molecular features between HGBL-DH/TH groups and the total DLBCL population allows identification of potentially useful screening features. In HGBL-DH/TH with BCL2 rearrangement, there was a significantly higher proportion of GCB (99% vs 50%) or GCB/unclassified by Lymph2Cx (100% vs 62%), GCB by IHC (99% vs 51%), CD10+ (95% vs 40%), BCL2+ (95% vs 68%), MUM1− (89% vs 44%), MYC+ (80% vs 48%), and DPE (75% vs 34%) compared with the total DLBCL cohort: all P < .001 (Figure 1B; supplemental Table 1). The 40% threshold for MYC positivity was originally defined based on correlation with clinical outcomes16 ; the impact of altering this threshold is shown in the supplemental material. Screening using a COO test would require performing FISH on 50% to 62% of the population (depending on the test used) and would allow detection of ≥99% of cases (Table 1). Meanwhile, using DPE as a screening tool would restrict FISH testing to a smaller population (34% of DLBCL tumors), resulting in a PPV of 0.14, but would result in missing 25% of cases. Combining DPE with GCB subtype (and/or Unclassified) by Lymph2Cx or Hans algorithm would not significantly further reduce this sensitivity but would restrict FISH to 11% to 14% of DLBCL, increasing the PPV to 30% to 37%. Results in the total HGBL-DH/TH group are similar because the vast majority have BCL2 rearrangement (Table 1). In HGBL-DH with BCL6 rearrangement, only the proportion of tumors that were MYC+ by IHC was significantly higher than the total cohort (87% vs 48%, P < .01), with no significant differences in other characteristics tested. Table 1 and supplemental Table 2 display the test characteristics of various screening strategies.
The only method that detects all cases of HGBL-DH/TH among tumors with DLBCL morphology is performance of MYC rearrangement testing in all cases, and then testing for BCL2 and BCL6 rearrangements where the FISH detected MYC rearrangement. HGBL-DH/TH with BCL2 rearrangement occurs almost exclusively in GCB DLBCL, allowing the screening of tumors for FISH testing by a COO test, albeit requiring FISH testing in more than one-half of tumors. Where resources are limited, combining COO with DPE would greatly reduce FISH testing, but at the expense of a moderate level of misclassification. We were unable to define a satisfactory screening strategy for the rare cases of HGBL-DH with BCL6 rearrangement.
The large size of this study allows accurate estimation of incidences and the application of FISH to all biopsies mitigates the bias of selective testing; however, there are caveats potentially affecting generalizability of the findings. First, the majority of the biopsies were incisional because core needle biopsies could not be included in TMA, which may bias against cases occurring at surgically inaccessible sites. Second, results from TMA may not accurately reflect results from tissue sections. Third, all of the biopsies involved in this study were from adult patients being considered for treatment with curative intent, potentially leading to underrepresentation of frail or seriously ill patients. Fourth, this study used MYC break-apart assays, which may miss a small proportion (<5% [James R. Cook, Cleveland Clinic, e-mail, 25 January 2018]) of MYC rearrangements only detectable using MYC/IGH dual fusion assays.7,19,20 Finally, FISH and IHC tests were performed in large academic centers and scored by expert hematopathologists; the accuracy of the testing may not be reliably reproducible in other settings.
Ultimately, decisions regarding the diagnostic workflow of tumors with DLBCL morphology will depend on laboratory resources, test prioritization when tissue is limited, and pathologist/physician preferences. The degree to which classification into HGBL-DH/TH alters clinical management will be a major factor.8,21 The results of this project, in concert with these factors, can inform decisions about whether and how to adopt screening tests, allow rational design of screening algorithms, and provide estimation of the impact of the screening implementation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Studies performed at the British Columbia Cancer Agency were supported by a Project Program Grant from the Terry Fox Research Institute (1023) and funding from the British Columbia Cancer Foundation, Genome Canada, and Genome BC. A.M. was supported by research fellowships from the German Cancer Aid, the Michael Smith Foundation for Health Research, and Lymphoma Canada. A portion of this study was coordinated by the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (Peter J. O'Dwyer and Mitchell D. Schnall, group cochairs) and supported by grants from the National Institutes of Health, National Cancer Institute (CA180820, CA180794, CA180790, CA180833, and CA180799).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: D.W.S. designed the research, performed research, analyzed data, and wrote the paper; R.L.K., A.M.S., S.B.-N., H.H., A.M., P.F., G.W.S., D.E., R.D.G., and G.O. performed research; A.J. performed statistical analyses; N.S., M.P., G.S.N., B.S.K., and J.M.C. contributed vital data; W.R.M. and A.R. designed the research and performed research; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: D.W.S has performed consultancy for Janssen and Celgene. B.S.K. has performed consulting for, and received research funding from, Genentech and Celgene. D.W.S., J.M.C., R.D.G., G.O., and A.R. are named inventors on a patent that has been licensed to NanoString Technologies. The remaining authors declare no competing financial interests.
Correspondence: David W. Scott, British Columbia Cancer Research Centre, 675 West 10th Ave, Room 12-114, Vancouver, BC V5Z 1L3, Canada; e-mail: dscott8@bccancer.bc.ca.
References
Author notes
W.R.M. and A.R. contributed equally to this study.