Key Points
GPVI aids in local immunity in gram-negative pneumonia–derived sepsis.
GPVI, CLEC2, and neutrophils do not play a major role in vascular integrity during pneumosepsis.
Abstract
Platelet collagen receptor glycoprotein VI (GPVI) and podoplanin receptor C-type lectin-like receptor 2 (CLEC2) are receptors implicated in platelet activation that both signal via an immunoreceptor tyrosine–based activation motif. Platelets are necessary for host defense and prevention of hemorrhage during sepsis, but the role of platelet GPVI and CLEC2 herein is unknown. To investigate this, we infected mice depleted of platelet GPVI or CLEC2 by antibody treatment or GPVI−/− mice with the common human sepsis pathogen Klebsiella pneumoniae via the airways to induce pneumonia-derived sepsis. The GPVI ligand collagen and the CLEC2 ligand podoplanin were constitutively present in the lung, whereas the GPVI ligands fibrin and histone were induced during pneumonia. During late-stage infection, both mice depleted of GPVI and GPVI−/− mice showed increased bacterial growth in lungs, and GPVI−/− mice also showed increased bacterial growth in distant body sites. Despite higher bacterial loads, GPVI-depleted mice showed reduced platelet numbers, platelet activation, and platelet–leukocyte complex formation in the bronchoalveolar space. Consistently, in human whole blood, GPVI stimulation of platelets increased platelet–leukocyte complex formation and leukocyte activation, which was accompanied by enhanced phagocytosis of Klebsiella. GPVI-depleted mice showed increased lung hemorrhage during infection, but not to the extent observed in platelet-depleted mice, and lung bleeding was not significantly different between GPVI−/− and wild-type mice. CLEC2 depletion did not affect any of the responses during pneumonia. These results suggest that platelet GPVI, but not CLEC2, contributes to local host defense during pneumonia-derived sepsis by enhancing leukocyte function.
Introduction
The essential role of platelets in primary hemostasis and limiting blood loss upon disruption of the vascular integrity has been well established.1 Platelets express multiple receptors through which they can be activated by a variety of ligands. Platelet receptors glycoprotein VI (GPVI) and C-type lectin-like receptor 2 (CLEC2) both signal via an immunoreceptor tyrosine–based activation motif (ITAM).2,3 GPVI is an immunoglobulin superfamily receptor and the major signaling receptor for collagen,4 but it can also be activated by laminin,5,6 adiponectin,7 fibrin,8 and histones.9 CLEC2 can be triggered by podoplanin and snake venom rhodocytin.10 Platelet GPVI is known for its role in thrombus formation,4 and CLEC2 is crucial for separation of lymphatic and blood vasculatures during embryonic development.10
During sterile inflammation (such as that induced by immune complexes), platelets prevent hemorrhage. In this setting, platelet GPVI, CLEC2, and ITAM signaling mediate sealing of neutrophil-induced vascular breaches via platelet recruitment, adhesion, and activation.11,12 Platelets also prevent infection-induced hemorrhage,13,14 but through which pathways is unknown. During bacterial infection, platelets in addition contribute to host defense.13-16 Platelets can limit bacterial growth and dissemination in experimental sepsis13-16 and can influence cytokine responses.13,14,17 The role of platelet GPVI and CLEC2 herein has not been investigated and remains to be elucidated.
We here aimed to determine the role of platelet GPVI and CLEC2 in the host response and prevention of hemorrhage during pneumonia-derived sepsis. We used a model of pneumonia and sepsis with the common human gram-negative sepsis pathogen Klebsiella pneumoniae, following the clinical scenario of a gradually growing bacterial load in the lungs with subsequent dissemination to distant body sites resulting in sepsis.
Materials and methods
Animals
Specific-pathogen-free C57Bl/6 mice (Charles River, Lyon, France) were housed in the Animal Research Institute Amsterdam facility under standard care. GPVI-deficient (GPVI−/−) mice and wild-type control mice, both on a C57Bl/6 background, were bred in the Institute of Experimental Biomedicine, University Hospital Würzburg, Würzburg, Germany. All experiments were conducted with female mice 8 to 12 weeks of age. The Institutional Animal Care and Use Committee of the Academic Medical Center approved all experiments.
Experimental study design
Pneumonia and sepsis was induced by intranasal inoculation with K pneumoniae serotype 2 (ATCC 43816, Rockville, MD; 10 000 colony-forming units [CFUs]) as described previously.13,18,19 Five days before induction of pneumonia, mice were injected IV with 1 µg/g body weight rat anti-mouse GPVI antibody JAQ-1 or control immunoglobulin G (IgG) (both from Emfret Analytics, Eibelstadt, Germany).20 Alternatively, mice were injected 5 and 2 days before infection with INU-1 antibody21 or control IgG (Emfret) (4 µg/g and 2 µg/g body weight). Mice were killed 12, 36, or 42 hours after induction of pneumonia (n = 8 per group); noninfected mice were sacrificed simultaneously (n = 4 per group). Time points were selected based on earlier publications with infection largely restricted to the lung at t = 12 hours with subsequent dissemination to distant body sites and sepsis at t = 36 and 42 hours.13,18,19 Lungs for pathology and bronchoalveolar lavage fluid (BALF) were obtained in separate experiments to avoid dilution of samples as previously described.22 In some experiments, mice were treated IV with a platelet-depleting antibody (anti-mouse-GPIb α, 2 µg/g body weight, Emfret) and with either neutrophil-depleting anti-mouse-GR1 (clone RB6.8C5; rat IgG IgG2b,κ) or its isotype control antibody anti-phytochrome (clone AFRC Mac 5.1; rat IgG IgG2b,κ) (both 100 µg), infected with K pneumoniae, and sacrificed after 12 hours; later time points were not studied, because neutrophil depletion results in early lethality in this model (data not shown). Bacterial quantification and storage of lung, blood, liver, and spleen were performed as previously described.13
Flow cytometry
For details regarding flow cytometry, see supplemental Methods and supplemental Figure 1 (available on the Blood Web site).
Protein measurements, ex vivo stimulations, and pathology
For details, see supplemental Methods.
Statistical analysis
Data are expressed as bars with 95% confidence intervals or as box-and-whisker-plots. Comparisons between groups were first performed using a (nonparametric) Kruskal-Wallis test; only when significant differences were present, groups at individual time points were tested using the Mann-Whitney U test. Analyses were done using GraphPad Prism 5.01 (GraphPad Software, San Diego, CA). P values < .05 were considered statistically significant.
Results
Ligands of GPVI and CLEC2 are abundantly present in the lung
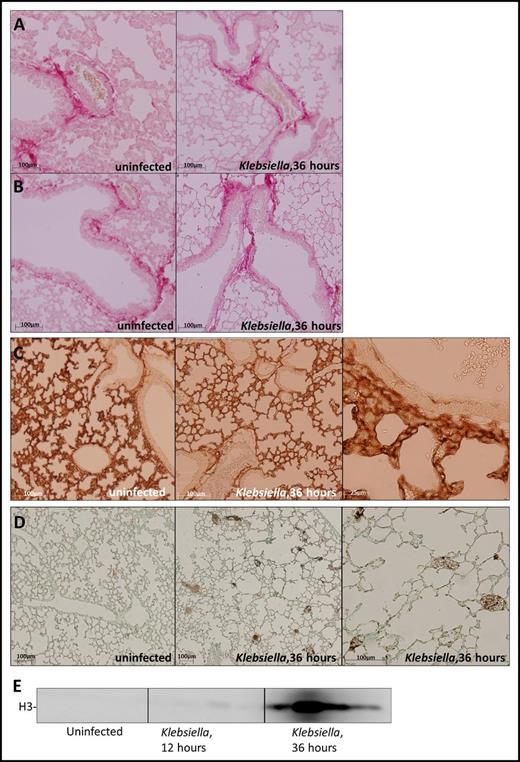
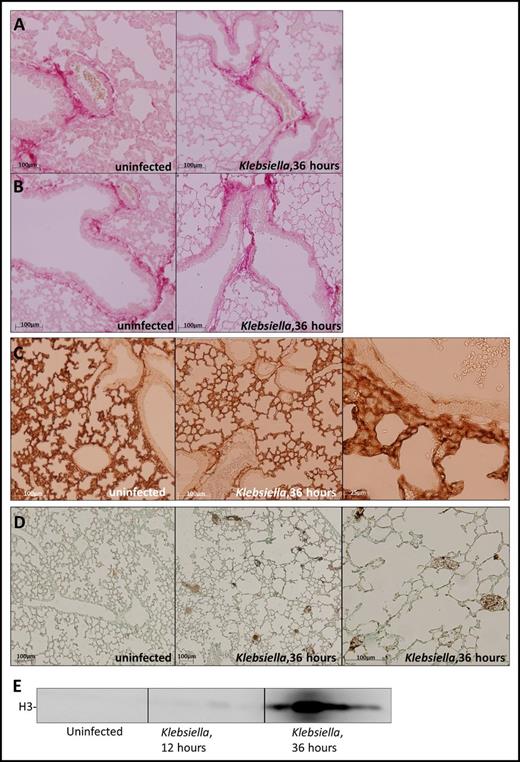
The major ligands for platelet GPVI and CLEC2 are collagen and podoplanin, respectively.2 Collagen is exposed during vessel wall damage; podoplanin is constitutively expressed by type 1 alveolar cells10 and can be upregulated on macrophages during infection.23 To assess the expression or GPVI and CLEC2 ligands in the lung during pneumonia and sepsis, we infected mice with common human sepsis pathogen K pneumoniae via the airways and stained lung sections for collagen and podoplanin before and after infection. Collagen was present in the lung lining the vessel wall (Figure 1A) and bronchial epithelial cells (Figure 1B) in uninfected and infected mice. Podoplanin was abundantly present in lungs in uninfected and infected mice, consistent with the fact that type I cells cover ∼95% of the alveolar surface (Figure 1C). Besides collagen, platelet GPVI can also be activated by fibrin or histones.8,9 Although these GPVI ligands were not detected in uninfected lungs, infection induced pulmonary fibrin deposition (Figure 1D) and also resulted in increased histone 3 levels in the BALF (Figure 1E). Akin to increasing fibrin levels during late-stage infection,24 histone 3 levels peaked 36 hours after infection (Figure 1E). These data show that ligands of GPVI and CLEC2 are abundantly present in the lung, partially constitutively and partially induced by infection.
Expression of platelet GPVI and CLEC2 ligands in the lung before and after induction of pneumonia. Mice were infected with K pneumoniae via the airways and sacrificed at the time point indicated; uninfected mice served as controls. (A-B) Picrosirius red staining of lung sections shows collagen underneath the vascular endothelium (A) and bronchial epithelial cells (B). (C) Podoplanin staining of lung sections. (D) Fibrin staining of lung sections. (E) Histone 3 levels in the BALF determined by western blot (n = 4 per group). Histology slides are representative of 4 mice per group.
Expression of platelet GPVI and CLEC2 ligands in the lung before and after induction of pneumonia. Mice were infected with K pneumoniae via the airways and sacrificed at the time point indicated; uninfected mice served as controls. (A-B) Picrosirius red staining of lung sections shows collagen underneath the vascular endothelium (A) and bronchial epithelial cells (B). (C) Podoplanin staining of lung sections. (D) Fibrin staining of lung sections. (E) Histone 3 levels in the BALF determined by western blot (n = 4 per group). Histology slides are representative of 4 mice per group.
Platelet GPVI supports local host defense during pneumonia-derived sepsis, whereas CLEC2 has no role
Platelets are necessary for adequate host defense during pneumosepsis.13 To determine the role of platelet GPVI and CLEC2 herein, we sought to deplete these receptors from the surface of platelets by administration of JAQ1 and INU-1, respectively, before induction of pneumonia-derived gram-negative sepsis. In accordance with previous reports,20,21,25 injection of JAQ1 and INU-1 completely depleted platelet GPVI and CLEC2, respectively (supplemental Figure 2). Platelet GPVI or CLEC2 depletion was sustained, lasting until at least 42 hours after infection (latest time point). JAQ1 and INU-1 administration can be associated with a transient thrombocytopenia,20,21 but because the antibody was injected several days before the start of the infection experiments, platelet counts were not decreased at the time of inoculation of bacteria compared with control mice (supplemental Figure 3). Sepsis did not alter expression of platelet GPVI or CLEC2 in mice (supplemental Figure 2).
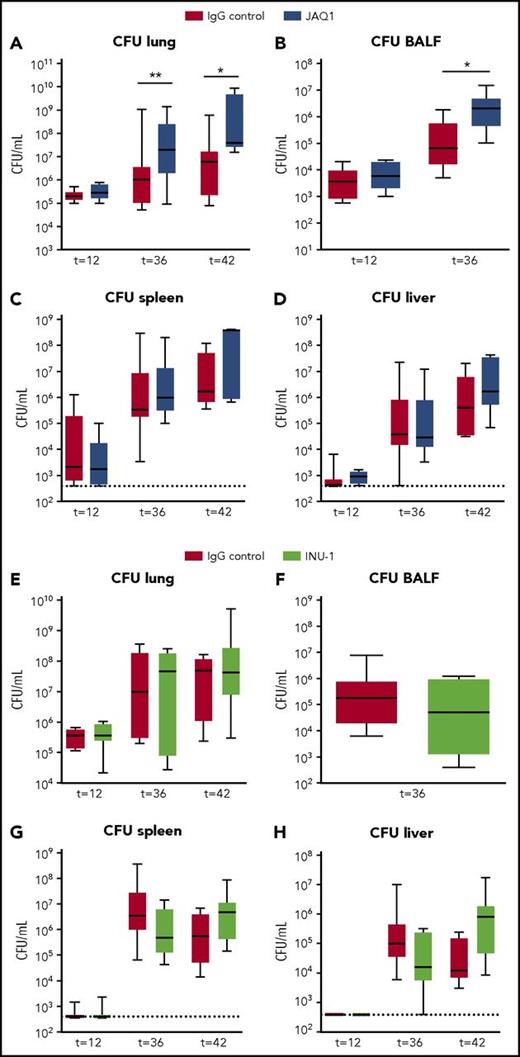
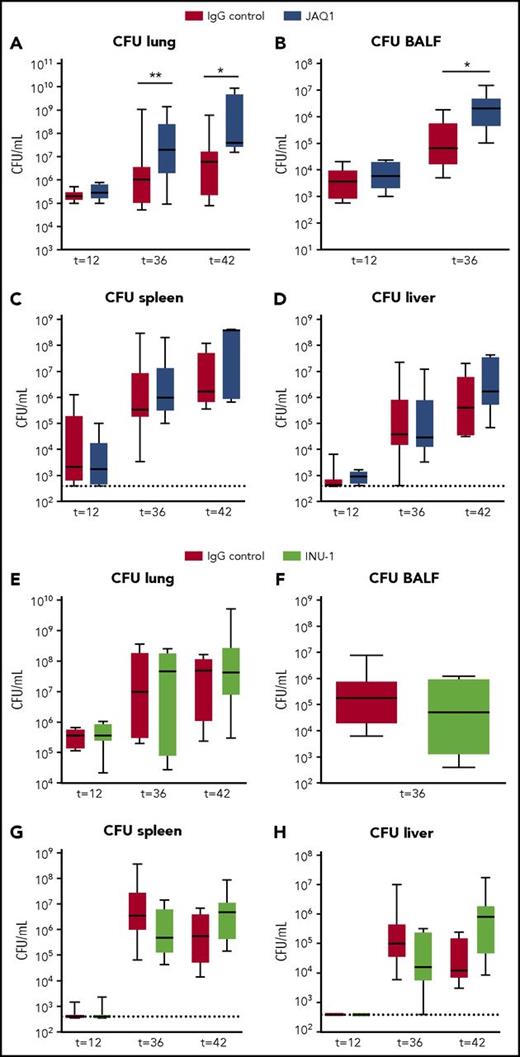
To study the role of platelet GPVI and CLEC2 in antibacterial defense, we quantified bacterial loads in mice depleted of platelet GPVI (JAQ1 treated) or CLEC2 (INU-1 treated) infected with K pneumoniae via the airways. Mice depleted of GPVI showed increased bacterial loads in the lung and BALF compared with control mice 36 and 42 hours after infection (Figure 2A-B). Platelet GPVI depletion only influenced local host defense, as bacterial loads in distant organs were similar between groups (Figure 2C-D). In mice depleted of CLEC2 host defense was not affected, as demonstrated by similar bacterial loads in lungs and distant organs (Figure 2E-H). These data indicate that platelet receptor GPVI aids in local host defense, whereas platelet CLEC2 had no role herein.
Platelet GPVI, but not CLEC2, supports local host defense during pneumonia-derived sepsis. Mice were injected with JAQ1 (5 days prior to infection), INU-1 (5 and 2 days prior to infection), or IgG control antibody, infected with K pneumoniae via the airways, and euthanized 12, 36, or 42 hours after infection. Data are number of CFUs in the body site indicated for JAQ1 (A-D) and INU-1 (E-H). Data are represented as box and whisker plots of 8 mice per group at each time point. *P < .05, **P < .005 vs IgG control.
Platelet GPVI, but not CLEC2, supports local host defense during pneumonia-derived sepsis. Mice were injected with JAQ1 (5 days prior to infection), INU-1 (5 and 2 days prior to infection), or IgG control antibody, infected with K pneumoniae via the airways, and euthanized 12, 36, or 42 hours after infection. Data are number of CFUs in the body site indicated for JAQ1 (A-D) and INU-1 (E-H). Data are represented as box and whisker plots of 8 mice per group at each time point. *P < .05, **P < .005 vs IgG control.
Platelet GPVI modulates local lung inflammation
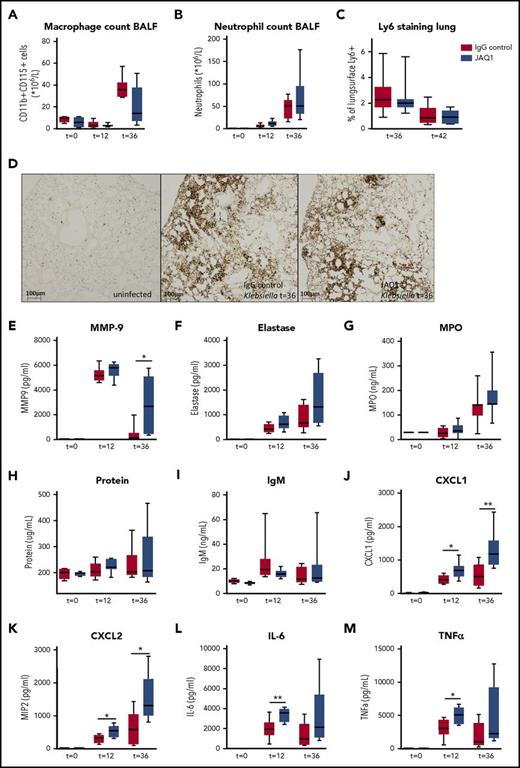
To assess the effect of GPVI or CLEC2 on local and systemic inflammatory responses, we first assessed leukocyte recruitment and functions. Macrophage and neutrophil counts in the BALF (Figure 3A-B) and neutrophil influx in lung tissue (Figure 3C-D) were not different between mice depleted of GPVI and control mice at any time point. To determine neutrophil activation in the airspace, we assessed several markers of neutrophil degranulation in the BALF. Matrix metalloproteinase 9 (MMP9) levels were significantly increased in the BALF in GPVI-depleted mice 36 hours after infection (Figure 3E; P < .05 vs IgG control), with a similar trend seen for elastase and myeloperoxidase (MPO), despite similar protein loads and IgM protein levels (Figure 3F-I). Also, levels of CXC chemokines were increased in the BALF of GPVI-depleted mice 12 and 36 hours after infection (Figure 3J-K; P < .05 vs IgG control). Moreover, local proinflammatory cytokine concentrations were increased 12 and 36 hours after infection, with higher levels of tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) in the BALF (Figure 3L-M; P < .05 vs IgG control). Together, these data suggest that platelet GPVI depletion increases MMP9, cytokine, and chemokine release in the bronchoalveolar space during pneumonia. No differences in any of these lung inflammatory responses were found between CLEC2-depleted mice and control mice (supplemental Figure 4).
Platelet GPVI modulates inflammation during pneumonia-derived sepsis. Mice were injected with JAQ1 or IgG control antibody, infected with K pneumoniae via the airways, and sacrificed 12, 36, or 42 hours after infection. (A) Macrophage counts in the BALF. (B-D) Neutrophil recruitment to the lung, measured by Ly6+ cell counts in the BALF (B) and Ly-6 staining of lung sections (C-D). (E-G) Neutrophil granulation markers in the BALF (E, MMP9; F, elastase, and G, MPO). (H) Protein levels in the BALF. (I) IgM levels in the BALF. (J-M) CXC chemokines and proinflammatory cytokines in the BALF (J, CXCL1; K, CXCL2; L, IL-6, and M, TNF-α). Data are presented as box and whisker plots of 8 mice per group at each time point. *P < .05, **P < .005 vs IgG control.
Platelet GPVI modulates inflammation during pneumonia-derived sepsis. Mice were injected with JAQ1 or IgG control antibody, infected with K pneumoniae via the airways, and sacrificed 12, 36, or 42 hours after infection. (A) Macrophage counts in the BALF. (B-D) Neutrophil recruitment to the lung, measured by Ly6+ cell counts in the BALF (B) and Ly-6 staining of lung sections (C-D). (E-G) Neutrophil granulation markers in the BALF (E, MMP9; F, elastase, and G, MPO). (H) Protein levels in the BALF. (I) IgM levels in the BALF. (J-M) CXC chemokines and proinflammatory cytokines in the BALF (J, CXCL1; K, CXCL2; L, IL-6, and M, TNF-α). Data are presented as box and whisker plots of 8 mice per group at each time point. *P < .05, **P < .005 vs IgG control.
Platelet GPVI is involved in platelet recruitment and activation and platelet–leukocyte complex formation in the lung, whereas CLEC2 has no role
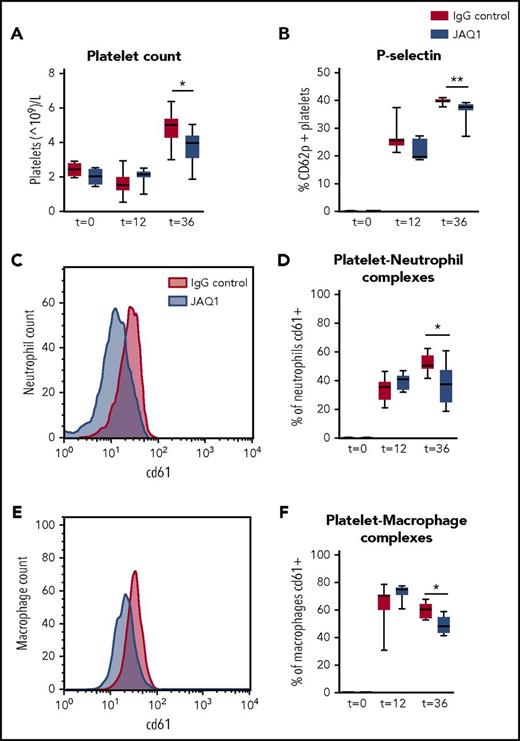
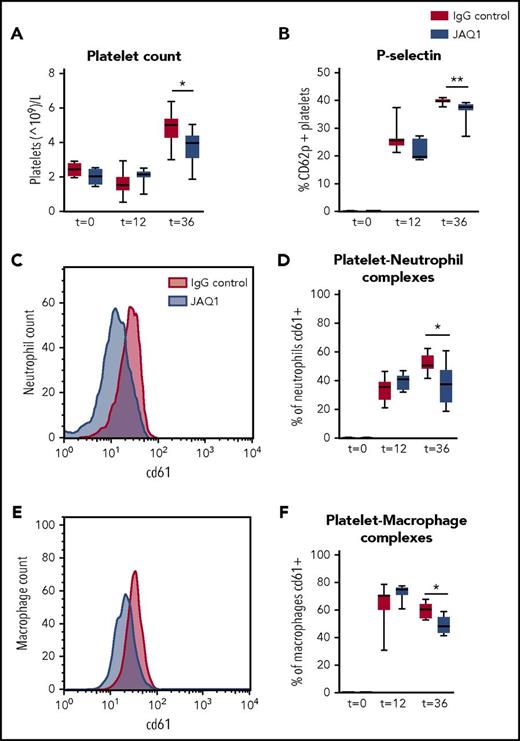
Platelet GPVI has been described to be involved in platelet recruitment to the site of inflammation.11 During pneumonia, platelet counts increased in the BALF but to a lower extent in GPVI-depleted mice (Figure 4A; P < .05 vs IgG control). Moreover, GPVI-depleted mice showed reduced platelet activation (P-selectin expression; Figure 4B; P < .005 vs IgG control) and reduced platelet–neutrophil and platelet–macrophage complex formation in the BALF (Figure 4C-F; P < .05 vs IgG control). Sepsis led to thrombocytopenia and platelet activation in peripheral blood (as reflected by enhanced P-selectin and CD63 expression), but this was not influenced by GPVI depletion, suggesting that platelet GPVI only influenced platelet functions at the primary site of infection (supplemental Figure 5). CLEC2 depletion did not modify any of the evaluated platelet responses in either the bronchoalveolar space or blood (supplemental Figure 6).
Platelet GPVI is involved in platelet influx and activation and platelet–leukocyte complex formation in the lung. Mice were injected with JAQ1 or IgG control antibody, infected with K pneumoniae via the airway, and sacrificed 12, 36, or 42 hours after infection. (A) Platelet counts. (B) Platelet P-selectin expression. (C-D) Platelet–neutrophil complex formation. (E-F) Platelet–macrophage complex formation. (C,E) Representative histogram of CD61 expression on neutrophils (C) of macrophages (E) in the BALF. Data are presented as box and whisker plots of 8 mice per group. *P < .05, **P < .005 vs IgG control.
Platelet GPVI is involved in platelet influx and activation and platelet–leukocyte complex formation in the lung. Mice were injected with JAQ1 or IgG control antibody, infected with K pneumoniae via the airway, and sacrificed 12, 36, or 42 hours after infection. (A) Platelet counts. (B) Platelet P-selectin expression. (C-D) Platelet–neutrophil complex formation. (E-F) Platelet–macrophage complex formation. (C,E) Representative histogram of CD61 expression on neutrophils (C) of macrophages (E) in the BALF. Data are presented as box and whisker plots of 8 mice per group. *P < .05, **P < .005 vs IgG control.
Platelet GPVI or CLEC2 inhibition does not impair activation of coagulation
Inflammation can enhance coagulation, and activation of coagulation can influence host defense.26 Platelets have been implicated in coagulation by affording a phospholipid surface for the assembly of activated clotting factors. Moreover, fibrin and d-dimer are ligands for GPVI.8 Therefore, we assessed activation of coagulation by assessing d-dimer formation in the lung. Infection induced activation of coagulation in the lung,13 but GPVI depletion did not impair this (supplemental Figure 7). Lung d-dimer levels were even higher in GPVI-depleted mice than in controls at 36 hours, most likely because of increased bacterial loads (supplemental Figure 7; P < .005 vs IgG control). GPVI depletion also did not impair systemic coagulation activation, as reflected by plasma thrombin–antithrombin complex concentrations, which were similar between groups (supplemental Figure 8). Activation of coagulation in lungs or plasma was not different between CLEC2-depleted mice and control mice (supplemental Figure 9; data not shown). These data show that platelet GPVI and CLEC2 are not necessary for activation of coagulation during gram-negative pneumosepsis.
Platelet GPVI or CLEC2 do not influence organ injury during Klebsiella sepsis
This model of pneumosepsis mimics clinical sepsis, as it is associated with organ injury during the later stages of infection13,19 (supplemental Figure 10). Depletion of platelet GPVI or CLEC2 did not affect lung or distant organ damage, as measured by pathology scores of lung and liver, lung weights, and levels of aminotransferase, alanine aminotransferase, and lactate dehydrogenase in plasma (supplemental Figure 10; data not shown). The fact that GPVI depletion did not influence lung damage was further supported by similar protein and IgM levels in the BALF (reflecting permeability; Figure 3H-I). Klebsiella infection also induced endothelial activation, as reflected by increased lung E-selectin levels, but these were also not different between groups (supplemental Figure 11).
Platelet GPVI stimulation modifies leukocyte functions in human whole blood
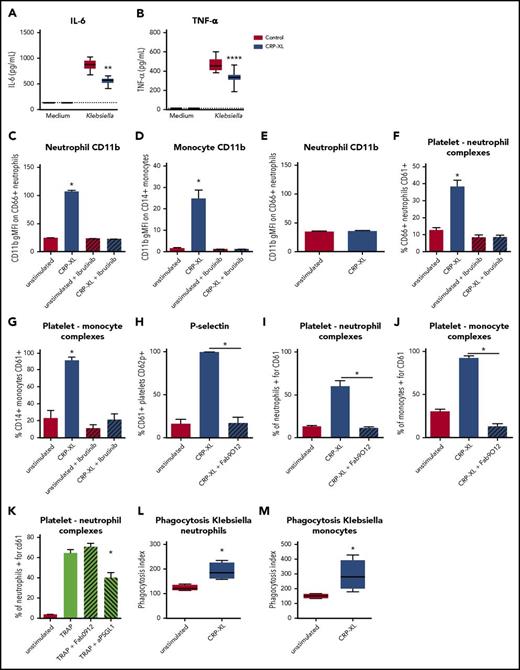
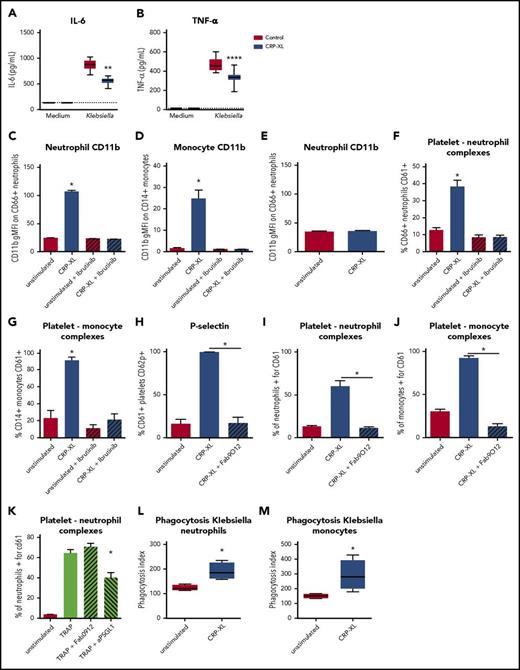
Depletion of platelet GPVI increased several inflammatory responses during pneumonia in mice, including MMP9, CXC chemokine, and cytokine release (Figure 3), and decreased platelet–leukocyte interactions (Figure 4). To assess whether GPVI also influences the inflammatory response in a human setting, we incubated human whole blood with the GPVI agonist CRP-XL27 and investigated the inflammatory reactions also analyzed in mice. This approach allows for assessment of the inflammatory response after a similar stimulatory dose, as in vivo differences in bacterial loads can also influence cytokine levels. In line with findings in vivo, GPVI activation by CRP-XL decreased Klebsiella-induced IL-6 and TNFα production (Figure 5A-B; P < .05 vs vehicle control) in human whole blood. In contrast, exposure of human blood to CRP-XL resulted in neutrophil and monocyte activation, as assessed by CD11b expression (Figure 5C-D; P < .05 vs unstimulated). CRP-XL did not stimulate neutrophils in the absence of platelets (Figure 5E), indicating that GPVI-activated platelets mediate neutrophil activation. GPVI signals via Bruton tyrosine kinase (BTK)2 and in accordance the BTK inhibitor ibrutinib28 prevented CRP-XL–induced leukocyte activation (Figure 5C-D). GPVI-stimulated platelets also bound more frequently to leukocytes, which was inhibited by ibrutinib (Figure 5F-G). It has recently been reported that platelet GPVI might be directly involved in platelet–neutrophil binding.11 To assess this, we used the GPVI-blocking Fab fragment Fab9012.29 Blocking GPVI completely abolished GPVI-mediated platelet activation and platelet–leukocyte complex formation (Figure 5H-J; P < .05 for CRP-XL + Fab9012 vs CRP-XL + vehicle control). Blocking GPVI, however, did not reduce platelet–neutrophil complex formation induced by stimulation of proteinase-activated receptor 1 using thrombin receptor–activating peptide (TRAPs).30 This TRAP-induced response was inhibited by blocking PSGL1 (Figure 5K). Moreover, we tested whether GPVI-mediated signaling could increase phagocytosis of Klebsiella and found that human whole blood stimulated with CRP-XL increased Klebsiella phagocytosis by neutrophils and monocytes (Figure 5L-M; P < .05 vs control). Together, these data show that platelet GPVI signaling can influence leukocyte functions in human blood.
GPVI stimulation in human whole blood decreases cytokine production but increases platelet–leukocyte complex formation, leukocyte activation, and Klebsiella phagocytosis. (A-B) Heparin human whole blood with collagen-related peptide (CRP-XLl 1 µg/mL) or vehicle control was incubated with UV-irradiated K pneumoniae (106 CFUs) for 4 hours, and plasma was stored for cytokine measurements (A, IL-6; B, TNF-α). (C-D) Human whole blood was stimulated with the GPVI agonist CRP-XL for 30 minutes in the presence or absence of the BTK inhibitor ibrutinib, and neutrophil (C) or monocyte (D) activation was assessed by CD11b expression. (E) Isolated neutrophils were stimulated with CRP-XL for 30 minutes, and neutrophil activation was assessed by CD11b. (F-G) Human whole blood was stimulated with CRP-XL for 30 minutes in the presence or absence of the BTK inhibitor ibrutinib, and platelet–neutrophil (F) or platelet–monocyte (G) complexes were determined by flow cytometry. (H-J) Human whole blood was stimulated with CRP-XL for 30 minutes with Fab9012 or vehicle control, and platelet activation (H) and platelet–neutrophil (I) or platelet–monocyte (J) complex formation was assessed by flow cytometry. (K) Human whole blood was stimulated with TRAP for 30 minutes in the presence of Fab9012 (blocking GPVI), anti-PSGL-1, or vehicle control, and platelet–neutrophil complex formation was assessed. (L-M) Human whole blood with or without CRP-XL was incubated at 37°C with fluorescein isothiocyanate–labeled heat-inactivated Klebsiella for 20 minutes, after which phagocytosis was determined in neutrophils (L) or monocytes (M). Data show representative results for 4 out of 6 donors (n = 8 replicates per group for cytokines, and n = 4 replicates per group for fluorescence-activated cell sorting readouts). Experiments were performed at least twice with different donors. *P < .05 for CRP-XL vs unstimulated, CRP-XL vs CRP-XL+Fab9012, and TRAP vs TRAP+aPSGL-1; **P < .01 and ****P < .0001 vs control.
GPVI stimulation in human whole blood decreases cytokine production but increases platelet–leukocyte complex formation, leukocyte activation, and Klebsiella phagocytosis. (A-B) Heparin human whole blood with collagen-related peptide (CRP-XLl 1 µg/mL) or vehicle control was incubated with UV-irradiated K pneumoniae (106 CFUs) for 4 hours, and plasma was stored for cytokine measurements (A, IL-6; B, TNF-α). (C-D) Human whole blood was stimulated with the GPVI agonist CRP-XL for 30 minutes in the presence or absence of the BTK inhibitor ibrutinib, and neutrophil (C) or monocyte (D) activation was assessed by CD11b expression. (E) Isolated neutrophils were stimulated with CRP-XL for 30 minutes, and neutrophil activation was assessed by CD11b. (F-G) Human whole blood was stimulated with CRP-XL for 30 minutes in the presence or absence of the BTK inhibitor ibrutinib, and platelet–neutrophil (F) or platelet–monocyte (G) complexes were determined by flow cytometry. (H-J) Human whole blood was stimulated with CRP-XL for 30 minutes with Fab9012 or vehicle control, and platelet activation (H) and platelet–neutrophil (I) or platelet–monocyte (J) complex formation was assessed by flow cytometry. (K) Human whole blood was stimulated with TRAP for 30 minutes in the presence of Fab9012 (blocking GPVI), anti-PSGL-1, or vehicle control, and platelet–neutrophil complex formation was assessed. (L-M) Human whole blood with or without CRP-XL was incubated at 37°C with fluorescein isothiocyanate–labeled heat-inactivated Klebsiella for 20 minutes, after which phagocytosis was determined in neutrophils (L) or monocytes (M). Data show representative results for 4 out of 6 donors (n = 8 replicates per group for cytokines, and n = 4 replicates per group for fluorescence-activated cell sorting readouts). Experiments were performed at least twice with different donors. *P < .05 for CRP-XL vs unstimulated, CRP-XL vs CRP-XL+Fab9012, and TRAP vs TRAP+aPSGL-1; **P < .01 and ****P < .0001 vs control.
Platelet GPVI, but not CLEC2, depletion modestly impairs vascular integrity in the lung during pneumonia
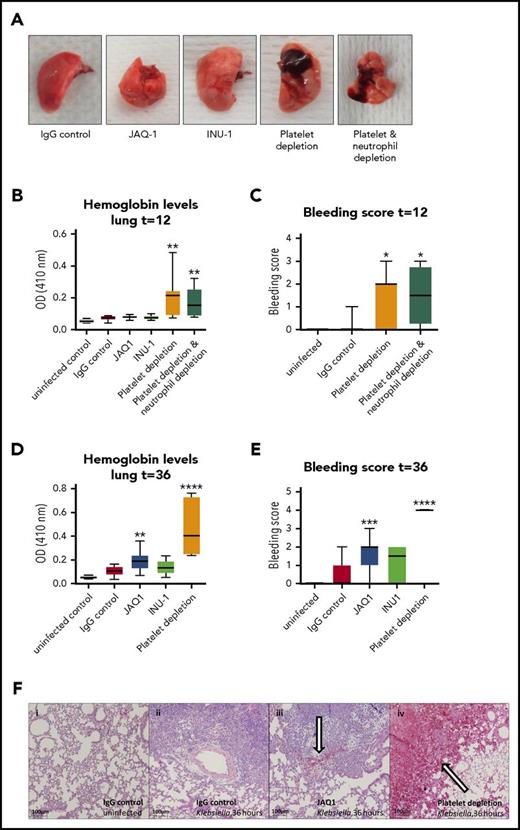
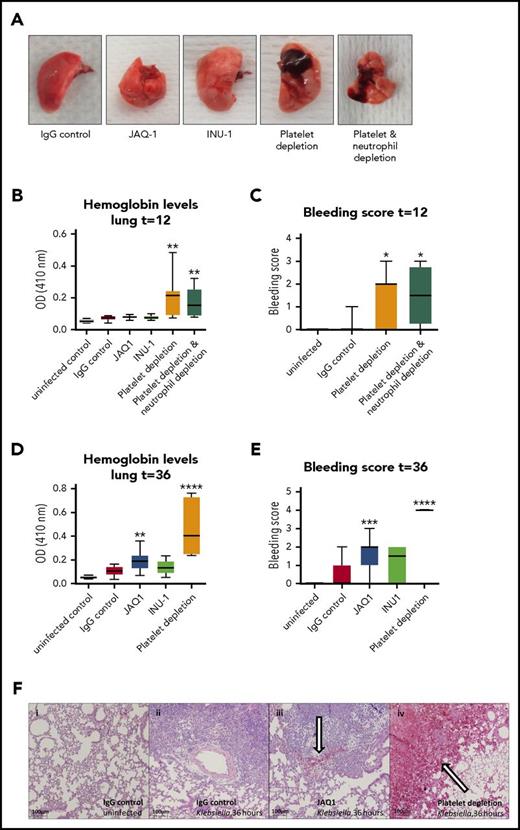
Platelets prevent bleeding during sterile inflammation,11,12,31,32 and our group has recently shown that platelets also prevent lung bleeding during pneumonia-derived sepsis.13 In models of sterile inflammation, platelet GPVI and CLEC2 are crucial in preventing bleeding.11,12 We therefore assessed whether lung bleeding occurred in GPVI- or CLEC2-depleted mice during Klebsiella pneumosepsis. In these studies, some groups were treated with either the platelet-depleting antibody anti-GPIb-α and/or the neutrophil-depleting antibody anti-Gr1, which in accordance with earlier reports reduced platelet counts to <1%13 and neutrophil counts to <10%33 of normal level, respectively. Consistent with previous findings,13 control mice did not shown bleeding in the lungs, whereas thrombocytopenic mice demonstrated pulmonary hemorrhage 12 hours after infection and to a larger extent at 36 hours, as assessed macroscopically, on hematoxylin and eosin–stained lung sections and by lung hemoglobin levels (Figure 6A-F). Although depletion of platelet GPVI did not result in lung hemorrhage early after infection (12 hours; Figure 6B), GPVI-depleted mice showed slightly increased bleeding 36 hours after infection compared with IgG controls (Figure 6D-F; P < .005 for lung hemoglobin levels and P < .0005 for lung bleeding score vs IgG control), but not to a similar extent as thrombocytopenic mice (Figure 6D-F; P < .001 for JAQ1 vs platelet depletion). CLEC2 depletion did not significantly induce lung bleeding.
Vascular integrity during pneumosepsis partly depends on platelet GPVI, but CLEC 2 or neutrophils are not crucially involved. Mice were injected with JAQ1, INU-1, or IgG control antibody or platelet-depleting antibody with or without depletion of neutrophils, infected with K pneumoniae via the airways, and sacrificed after 12, 36, or 42 hours after infection. (A-F) Panels show lung bleeding. (A) Representative photographs of infected lungs. Lung bleeding early after infection (t = 12; B-C) or at late-stage pneumosepsis (t = 36; D-F). (B,D) Hemoglobin was measured in 50-fold-diluted lung homogenates by light density at 410 nm optical density (OD). (C,E) Lung bleeding was scored on hematoxylin and eosin–stained tissue sections by a pathologist blinded for groups. (F) Representative photomicrographs of hematoxylin and eosin–stained lung sections of uninfected and infected mice at 36 hours. (i-iv) 40× enlarged pictures of uninfected mice receiving IgG control (i), 36-hour-infected IgG control (ii), JAQ1-treated mice (iii), or mice with a <1% platelet count due to aGPIb antibody (iv). Bleeding is indicated by an arrow. Data are presented as box and whisker plots of 8 mice per group at each time point. In panel E, all platelet-depleted mice had the maximal bleeding score (4). *P < .05, **P < .005, ***P < .0005, and ****P < .00005 vs IgG control.
Vascular integrity during pneumosepsis partly depends on platelet GPVI, but CLEC 2 or neutrophils are not crucially involved. Mice were injected with JAQ1, INU-1, or IgG control antibody or platelet-depleting antibody with or without depletion of neutrophils, infected with K pneumoniae via the airways, and sacrificed after 12, 36, or 42 hours after infection. (A-F) Panels show lung bleeding. (A) Representative photographs of infected lungs. Lung bleeding early after infection (t = 12; B-C) or at late-stage pneumosepsis (t = 36; D-F). (B,D) Hemoglobin was measured in 50-fold-diluted lung homogenates by light density at 410 nm optical density (OD). (C,E) Lung bleeding was scored on hematoxylin and eosin–stained tissue sections by a pathologist blinded for groups. (F) Representative photomicrographs of hematoxylin and eosin–stained lung sections of uninfected and infected mice at 36 hours. (i-iv) 40× enlarged pictures of uninfected mice receiving IgG control (i), 36-hour-infected IgG control (ii), JAQ1-treated mice (iii), or mice with a <1% platelet count due to aGPIb antibody (iv). Bleeding is indicated by an arrow. Data are presented as box and whisker plots of 8 mice per group at each time point. In panel E, all platelet-depleted mice had the maximal bleeding score (4). *P < .05, **P < .005, ***P < .0005, and ****P < .00005 vs IgG control.
Neutrophils have been identified as responsible for inducing hemorrhage during sterile inflammation.11,32 In contrast, neutrophil depletion did not abrogate bleeding in thrombocytopenic mice (Figure 6A-C). These results suggest that during infection, prevention of bleeding by platelets may partially be mediated by GPVI but does not rely on CLEC2 or neutrophils.
GPVI−/− mice also have impaired immunity during gram-negative pneumonia–derived sepsis
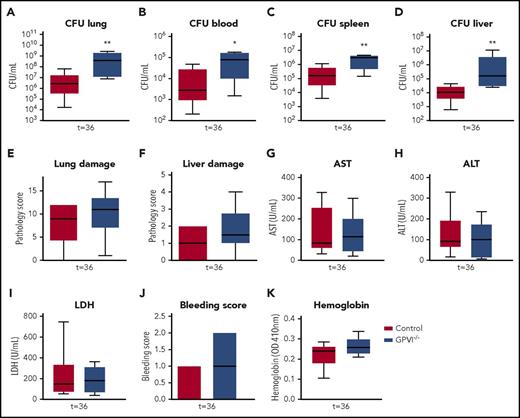
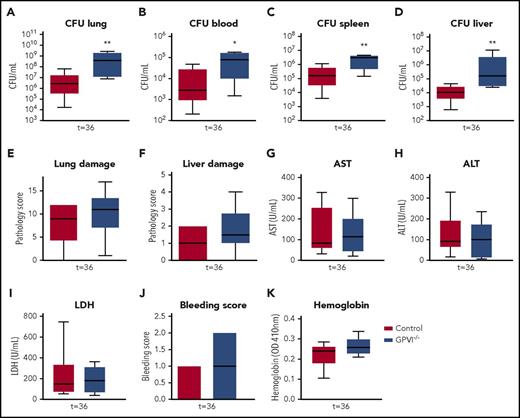
To exclude JAQ1 effects unrelated to GPVI depletion, validation experiments were performed in GPVI−/− mice. GPVI−/− and wild-type mice were infected with Klebsiella via the airways and killed after 36 hours (ie, at the time point where the effects of JAQ1-induced GPVI depletion were most clear). GPVI−/− mice showed no GPVI expression on platelets (supplemental Figure 12) and had platelet counts similar to those of wild-type mice (supplemental Figure 12). Like JAQ1-treated mice, GPVI−/− mice showed increased bacterial growth in the lungs compared with control mice (Figure 7A; P < .005 vs wild-type). GPVI−/− mice also had increased bacterial loads in blood and distant organs (Figure 7B-D; P < .05 vs wild-type). Akin to JAQ1-treated mice, the extent of lung pathology and distant organ damage was similar between groups (Figure 7E-I). The modestly increased bleeding during infection seen in JAQ1-treated mice was, however, even less pronounced in GPVI−/− mice when compared with controls and not significantly different (Figure 7J-K). These results confirm that platelet GPVI aids in host defense during Klebsiella pneumosepsis but argue against an important role for GPVI in protection of vascular integrity.
GPVI−/− mice also have impaired immunity during gram-negative pneumonia–derived sepsis. Control or GPVI−/− mice were infected with K pneumoniae via the airways and sacrificed 36 hours after infection. (A-D) Bacterial loads for organs indicated. (E) Lung pathology score. (F) Liver pathology scores. (G-I) Levels of aspartate aminotransferase (AST) (G), alanine aminotransferase (ALT) (H), and lactate dehydrogenase (LDH) (I). (J) Bleeding score in the lung. (K) Hemoglobin levels in the lung. Data are presented as box and whisker plots of 8 mice per group. *P < .05 and **P < .005 vs control.
GPVI−/− mice also have impaired immunity during gram-negative pneumonia–derived sepsis. Control or GPVI−/− mice were infected with K pneumoniae via the airways and sacrificed 36 hours after infection. (A-D) Bacterial loads for organs indicated. (E) Lung pathology score. (F) Liver pathology scores. (G-I) Levels of aspartate aminotransferase (AST) (G), alanine aminotransferase (ALT) (H), and lactate dehydrogenase (LDH) (I). (J) Bleeding score in the lung. (K) Hemoglobin levels in the lung. Data are presented as box and whisker plots of 8 mice per group. *P < .05 and **P < .005 vs control.
Discussion
Although recent studies have greatly improved our knowledge of the function of platelet receptors GPVI and CLEC2 in thrombosis and vascular integrity during noninfectious conditions,2,3,11,12 their role in sepsis has not been investigated. We here show that during gram-negative pneumosepsis, platelet GPVI influences local host defense and inflammation at the primary site of infection. In contrast, platelet CLEC2 depletion had no effect on the host response.
Several factors can possibly explain the different roles of GPVI and CLEC2 during pneumosepsis. First, GPVI and CLEC2 are activated by different agonists. Whereas the CLEC2 ligand podoplanin is constitutively expressed in lungs of uninfected mice, the GPVI ligands collagen, fibrin,8 and histones9 are mainly expressed in settings of vessel damage, coagulation, and/or inflammation, which are present during pneumonia. Because the main GPVI ligand collagen is located in the subendothelial layer, the binding and activation of platelets through GPVI must occur after exposure to the subendothelium at sites of inflammation, which may at least in part explain why platelet GPVI depletion selectively influenced local responses in the lung without affecting systemic reactions. Moreover, in contrast to CLEC2, multiple ligands can activate GPVI in the infected lung. Of note, although platelet GPVI and CLEC2 use similar signaling pathways, differences exist between signal transduction; whereas GPVI uses an ITAM containing the GPVI–FcRc chain complex, CLEC2 phosphorylates tyrosine within the hemITAM, both eventually leading to the activation of phospholipase Cγ22. Whether these differences contribute to the different phenotypes of GPVI- and CLEC2-depleted mice during pneumosepsis remains to be established.
Platelet GPVI has been reported to enhance leukocyte recruitment during glomerulonephritis34 and immune complex–mediated inflammation.11 Moreover, platelet GPVI can enhance platelet recruitment and activation during sterile inflammation.11 During pneumonia and sepsis, leukocyte counts increased in the bronchoalveolar space by a GPVI independent mechanism, in line with previous findings that platelet depletion did not impair leukocyte recruitment during Klebsiella pneumosepsis.13 This indicates that during Klebsiella infection, other factors can induce adequate leukocyte recruitment in the absence of platelets (function). However, platelet influx, as was platelet activation and platelet–neutrophil and macrophage complex formation, was reduced in GPVI-depleted mice. Platelets can potentiate leukocyte functions by secretion or direct binding. It therefore seems possible that either GPVI mediated increased binding at the site of infection or direct GPVI-mediated platelet activation potentiates leukocyte functions locally in the lung, resulting in increased host defense. In line with the latter possibility, we found that stimulation of GPVI in human whole blood increased platelet–leukocyte complex formation and leukocyte activation. Moreover, we found that GPVI-mediated platelet stimulation in human whole blood increased Klebsiella phagocytosis.
It has recently been reported that platelet GPVI can directly bind neutrophils.11 During pneumosepsis, thrombin-activated platelets could potentially directly use GPVI for neutrophil binding. However, using in vitro experiments, we found that blocking GPVI did not reduce neutrophil-platelet binding in TRAP-stimulated human whole blood. In the BALF of infected mice, GPVI inhibition reduced platelet–neutrophil complexes, which is therefore most likely not a consequence of reduced direct binding between GPVI and neutrophils.
In contrast to the effects on host defense, depletion of platelet GPVI increased chemokine and cytokine responses in vivo in mice, and conversely, GPVI activation decreased cytokine production in human whole blood, suggesting that platelet GPVI has an anti-inflammatory effect on leukocytes. Likewise, previous studies documented increased cytokine levels in platelet-depleted mice with sepsis13,35 or after high-dose IV lipopolysaccharide or Escherichia coli challenges.35,36 It seems that although platelets (when normally present) can aid in host defense against bacteria, they can also dampen leukocyte activation. These effects on leukocyte activation are in contrast to findings in a model of skin inflammation, where platelet GPVI increased MPO, MMP9, and IL-6 levels.11,37 Thus, platelet (GPVI)-induced inflammatory changes in monocytes/macrophages are likely dependent on stimuli and setting. Moreover, in contrast to the similar effect of GPVI signaling on cytokine levels in mice and humans during Klebsiella infection, the effect of GPVI signaling on neutrophil activation is more complex. Murine GPVI inhibition increased MMP9 release, indicative of neutrophil activation; however, GPVI stimulation in human whole blood increased CD11b expression, which is also indicative of neutrophil activation. These discrepancies might be due to differences in mice vs humans, stimuli, or regulation of granule secretion compared with integrin expression.
Activated platelets provide a negatively charged surface for the conversion of coagulation factors.38 Our group has previously reported that depletion of platelets does not impair activation of coagulation during murine sepsis.13 In line with this, we found that GPVI or CLEC2 depletion did not impair activation of coagulation during Klebsiella sepsis. GPVI-depleted mice even showed higher levels of d-dimer formation in the lung 36 hours after infection, most likely driven by higher bacterial loads. A recent study found that platelet CLEC2 was crucial during Salmonella-induced liver thrombosis.26 We found no effect of platelet CLEC2 on coagulation or thrombus formation. Differences might be explained by the different experimental setting; whereas the Salmonella infection was cleared and induced large thrombus formation,26 our model of Klebsiella pneumosepsis is associated with gradually increasing bacterial loads and more modest thrombus formation.
Previous studies using models of sterile inflammation (such as immune complex–mediated inflammation of the skin) have investigated how platelets protect against bleeding during inflammation.11,12,34 Platelets sealed neutrophil-induced breaches of the vessel wall, a process independent of thrombus formation11 but dependent on platelet GPVI and CLEC2.11,12 Our group has recently reported that during sepsis, platelets are also crucial for vascular integrity, with severe lung bleeding seen in thrombocytopenic mice with sepsis, but not in uninfected mice.13 During pneumosepsis, mice depleted of GPVI showed slightly more bleeding, but not to the extent of platelet depletion, whereas platelet CLEC2 depletion had no effect. However, in validation experiments with GPVI−/− mice, the extent of bleeding did not significantly differ between GPVI-deficient and wild-type mice during infection. The difference between data obtained with JAQ1 treatment and GPVI−/− mice could be related to possible GPVI-independent effects of JAQ1 and/or compensatory mechanisms in mice with a genetic deficiency for GPVI. Nonetheless, although both approaches to the study the role of GPVI have their limitations, they both indicate an important role for platelet GPVI in antibacterial defense and a modest function (if any) in maintaining vascular integrity during gram-negative pneumosepsis. In earlier investigations, we established that platelet P-selectin19 and Toll-like receptor signaling18 are not involved in maintaining the vascular integrity in the lung in this model of pneumosepsis. Likewise, inhibiting the platelet P2Y12 receptor by clopidogrel is not associated with lung hemorrhage in the current model (data not shown). It is possible that GPIb or proteinase-activated receptors might be involved in the vasculoprotective action of platelets during the early stages of pneumosepsis. Although neutrophil-dependent inflammation is responsible for the occurrence of bleeding during sterile inflammation,11,32 we here show that the extent of lung hemorrhage was similar between thrombocytopenic mice depleted of neutrophils and thrombocytopenic mice with normal neutrophil counts, indicating that neutrophils do not crucially mediate thrombocytopenic bleeding during sepsis. During infection, in comparison with sterile inflammation, damage might be more heterogeneous with multiple leukocyte subsets involved, such as monocytes, macrophages, or lymphocytes, whereas direct vascular damage by Klebsiella can also not be excluded. Together, these data suggest that the prevention of hemorrhage during infection is in part differentially regulated compared with sterile inflammation.
Several readouts showed variation between different experiments, most notably the number of CFUs and the extent of platelet activation, which most likely is related to the use of viable bacteria (which are freshly prepared for each experiment) and different batches of mice. Notably, however, each individual experiment comparing 2 groups at a predefined time point was strictly controlled in that all mice were infected at the same time with the exact same inoculum, allowing adequate analyses of group differences.
In conclusion, we demonstrate that platelet GPVI (but not CLEC2) supports host defense and modulates inflammation, platelet influx, activation, and platelet–leukocyte complex formation at the primary site of infection during gram-negative pneumonia–derived sepsis. Our findings that platelet GPVI, platelet CLEC2, and neutrophils do not play a major role in preventing bleeding in the lung during bacterial pneumonia exemplify differences in the mechanisms that maintain the vascular integrity at sites of inflammation in noninfectious and infectious conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Jandrot-Perrus (Institute National de la Santé et de la Recherche Médicale, Paris, France) for supplying Fab9012; J. B. Daalhuisen, M. ten Brink, and R. de Beer (Center for Experimental and Molecular Medicine, Academic Medical Center, Amsterdam) for technical support; and D. Stegner (Institute of Experimental Biomedicine, University of Würzburg, Würzburg, Germany) for help with GPVI−/− mice.
T.A.M.C. was funded by the Landsteiner Foundation for Blood Transfusion Research (grant 1351).
Authorship
Contribution: T.A.M.C., A.F.d.V., C.v.t.V., and T.v.d.P. designed the study; T.A.M.C acquired all the data. B.N. and L.B. helped with experimental setup; T.A.M.C., J.J.T.H.R., and O.J.d.B. analyzed the data; all authors were involved in the interpretation of the data; T.A.M.C. and T.v.d.P. drafted the manuscript; and all authors critically reviewed and revised manuscript for important intellectual content and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Theodora A. M. Claushuis, Center for Experimental and Molecular Medicine, Academic Medical Center, Meibergdreef 9, Room F0-117, 1105 AZ Amsterdam, The Netherlands; e-mail: t.a.claushuis@amc.uva.nl.