Key Points
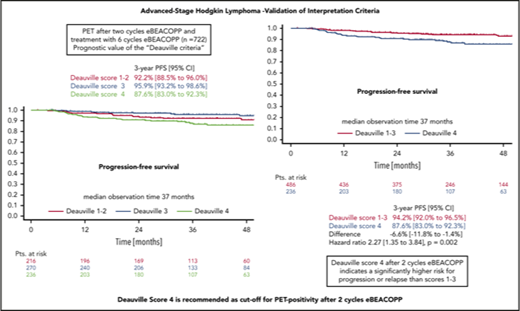
DS4 is recommended as the cutoff value for PET-2 positivity after 2 cycles eBEACOPP for advanced-stage HL.
Abstract
The HD18 study for patients with newly diagnosed advanced-stage Hodgkin lymphoma (HL) used positron emission tomography (PET) after 2 cycles (PET-2) of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated doses (eBEACOPP) to guide further treatment. Here, we analyzed the impact of PET-2 results in the context of eBEACOPP according to the Deauville score (DS) in patients treated within the HD18 trial. Residual tissue was visually compared with reference regions according to DS. We analyzed the association between PET-2 uptake and baseline characteristics, progression-free survival (PFS), and overall survival (OS). One thousand five patients (52%) had DS1 or DS2, 471 (24%) had DS3, and 469 (24%) DS4. PET-2 uptake was associated with baseline risk factors large mediastinal mass, extranodal disease, and high International Prognostic Score (P < .0001 each). Among 722 patients receiving standard therapy with 6 cycles of eBEACOPP, 3-year PFS rates were 92.2%, 95.9%, and 87.6% with DS1-2, DS3, and DS4, respectively. Univariate hazard ratio (HR) for PFS in patients with DS4 vs DS1-3 was 2.3 (1.3-3.8; P = .002). DS4 was the only factor remaining significant for PFS in a multivariate analysis including the associated baseline risk factors. Three-year OS rates were 97.6% for DS1-2, 99.0% for DS3, and 96.8% for DS4, with a univariate HR for DS4 vs DS1-3 of 2.6 (1.0-6.6; P = .04). Residual uptake above that in the liver at PET-2 (ie, DS4) is an important risk factor regarding survival outcomes for patients treated with eBEACOPP upfront. We thus recommend DS4 as the cutoff value for PET-2 positivity. This trial was registered at www.clinicaltrials.gov as #NCT00515554.
Introduction
Metabolic response assessed by 18F-fluorodeoxyglucose (FDG)–positron emission tomography (PET) after 2 cycles of chemotherapy (PET-2) has been successfully introduced into first-line therapy of advanced-stage classical Hodgkin lymphoma (HL).1-5 PET is used to tailor treatment intensity to the individual patient’s needs and thus helps to avoid undertreatment or overtreatment.6-14 The risk of treatment failure on the one hand and excessive toxicity on the other hand can thereby be reduced.
Interpretation of PET-2 scanning is a subject of ongoing debate. The Deauville score (DS) was introduced to ensure comparability between study groups and consistency of PET interpretation.15,16 By comparing the uptake within residual lymphoma tissue to the reference regions mediastinum and liver, a higher interreader concordance could be reached.17 Although the liver is nowadays often used as cutoff for a positive PET (≥DS4), we used the mediastinum as cutoff (≥DS3) in our German Hodgkin Study Group (GHSG) sixth-generation trials HD16-18. We used this conservative approach in order to avoid the risk of undertreatment when deescalating treatment intensity after a negative PET scan. The DS has so far mainly been applied and predominantly validated under doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) treatment. To identify the cutoff that is best suited to separate risk groups after 2 cycles of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated doses (eBEACOPP) in advanced-stage HL,18 we analyzed progression-free (PFS) and overall survival (OS) in patients who underwent standard treatment within the HD18 trial according to their PET-2 results.
Methods
Patients
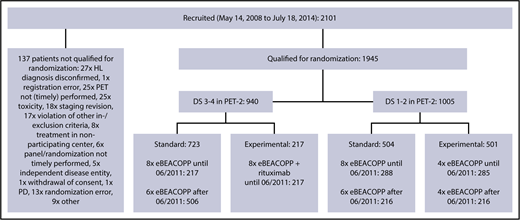
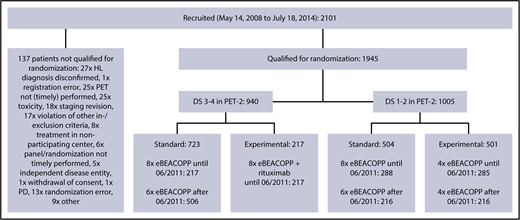
Between 14 May 2008 and 18 July 2014, a total of 2101 patients aged 18 to 60 years with newly diagnosed, biopsy-proven, advanced-stage HL from 5 European countries were included in the international phase 3 HD18 trial; they underwent PET-2–guided treatment including eBEACOPP (Figure 1). The study was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for good clinical practice, approved by the respective ethics committees. All patients provided written informed consent before study entry. Demographic and clinical data were obtained from the database of the trial’s final analysis performed in March 2017.
Trial profile. Between May 2008 and July 2014, a total of 1945 patients were included in the HD18 trial and received 2 cycles of eBEACOPP followed by PET-2. After a protocol amendment in 2011, the standard arm was modified from 8 to 6 cycles of eBEACOPP (=722 patients). PD, progressive disease.
Trial profile. Between May 2008 and July 2014, a total of 1945 patients were included in the HD18 trial and received 2 cycles of eBEACOPP followed by PET-2. After a protocol amendment in 2011, the standard arm was modified from 8 to 6 cycles of eBEACOPP (=722 patients). PD, progressive disease.
Imaging
All patients received 2 cycles of eBEACOPP followed by a restaging including PET-2 scan. A multidisciplinary panel of experts from medical oncology, nuclear medicine, radiation oncology, and radiology centrally reviewed all PET and computed tomography (CT) scans as well as radiographs and clinical information, and determined the interim DS,3 comparing the residual uptake to reference regions. Patients with progressive disease, denoted by DS5, were taken off protocol and excluded from further analysis. In the HD18 trial, an FDG uptake higher than the mediastinal uptake (DS3 and DS4) at PET-2 was defined as positive.
HD18 study design
Following central review, patients were randomly assigned to a treatment group based on their PET-2 result: before June 2011, patients were randomized in a 1:1 ratio between the standard arm 8 cycles of eBEACOPP in total and the experimental treatment depending on PET-2. Experimental treatment consisted of a total of 8 cycles of eBEACOPP combined with rituximab for PET-2+ patients and a total of 4 cycles of eBEACOPP for PET-2− patients. After June 2011, patients with a negative PET-2 (DS1 and DS2) were randomized 1:1 to a total of 6 cycles of eBEACOPP or 4 cycles of eBEACOPP, whereas all PET-2+ patients (DS3 and DS4) received a total of 6 cycles of eBEACOPP. Patients with progressive disease were taken off protocol. Radiotherapy to PET+ residual tissue after chemotherapy was recommended in all groups. Until April 2014, the mediastinum was used as reference region for the radiotherapy indication (DS3 and DS4). Following a protocol amendment, radiotherapy recommendation was later restricted to residual uptake higher than in the liver (DS4).14
Statistical analysis
The objectives of this retrospective analysis were to explore the association of FDG uptake within residual lymphoma tissue with baseline characteristics and treatment outcome, considering different cutoffs for PET-2 positivity.
For all statistical tests, the PET-2 interpretation was dichotomized using 2 different cutoffs: the more conservative cutoff used in the HD18 trial, where uptake higher than that in the mediastinum (DS3 and DS4) is defined as positive, vs the currently more common interpretation whereby an uptake higher than that in the liver (only DS4) is defined as positive.
We explored the association between residual uptake in PET-2 and baseline characteristics in all patients qualifying for randomization, by applying means of descriptive statistics, the Fisher exact test, and multivariate logistic regression models including all factors with univariate P < .001.
To ensure comparability, the impact of residual uptake in PET-2 on treatment outcomes was analyzed among those patients within the standard therapy arms of our trial after June 2011 (survival analysis set). PFS and OS were analyzed according to Kaplan-Meier, using Cox regression for comparisons. PFS was defined as the time from completion of staging until progression, relapse, or death from any cause, or to the day when information was last received on the patient’s disease status. OS was defined as time from completion of staging until death from any cause, or until the day information was last received with regard to the patient’s being alive. To account for a possible influence of baseline risk factors on our results, we calculated multivariate regression models including the associated factors, large mediastinal mass, extranodal involvement, and International Prognostic Score (IPS). All statistical computations were performed with SAS 9.4 (SAS Institute, Cary, NC).
Results
PET-2 uptake and baseline characteristics
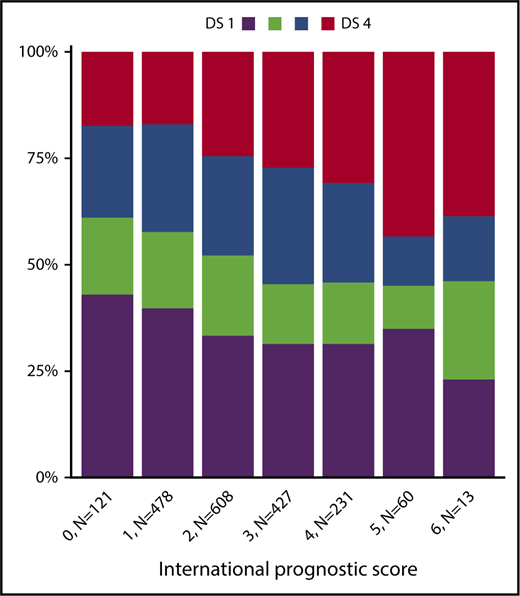
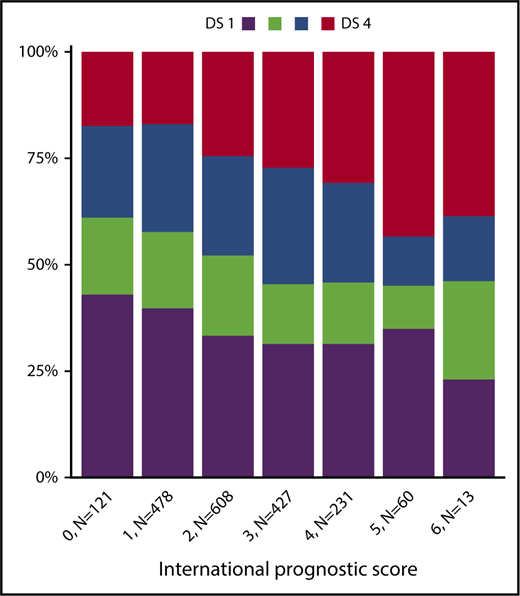
Among 1945 patients who underwent PET-2 within the HD18 trial (Figure 1), 1005 (52%) had a residual tissue uptake not higher than that in the mediastinum (DS1 or DS2), 471 (24%) had an uptake higher than the mediastinum but not higher than in the liver (DS3), and 469 (24%) had an uptake higher than in the liver (DS4). There was a strong association between PET-2 results and the initial baseline characteristics large mediastinal mass, extranodal involvement, and high IPS > 2 (Table 1; Figure 2). Only 1 patient showed progressive disease under eBEACOPP and did not qualify for PET-2 randomization; we did not identify any other patient who might have qualified as DS5, according to its definition as ”markedly increased uptake at any site and new sites of disease.”15
PFS
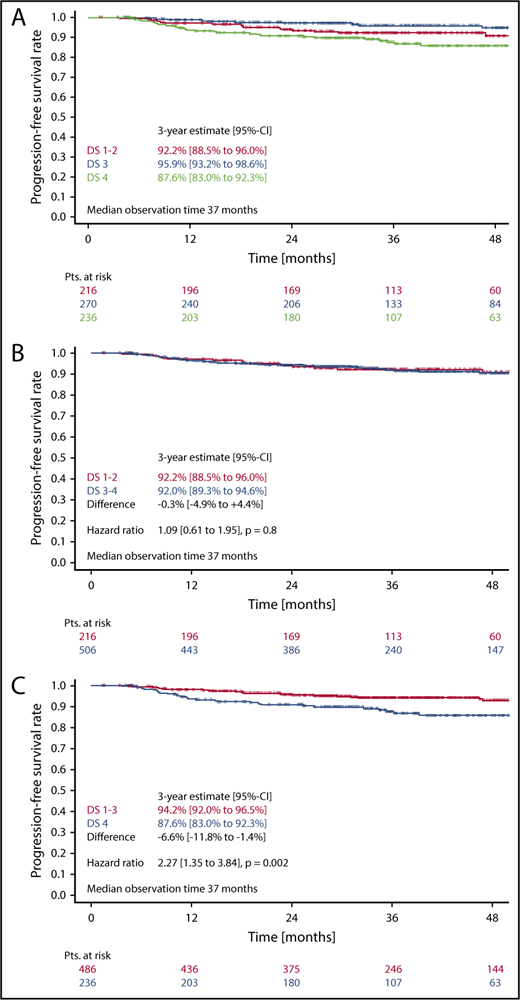
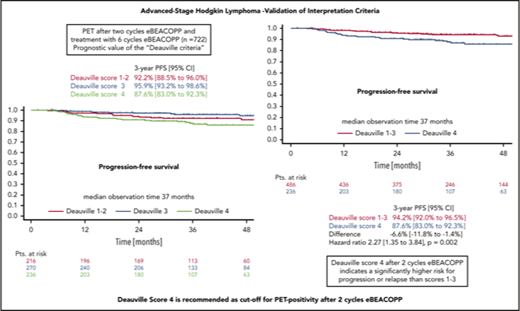
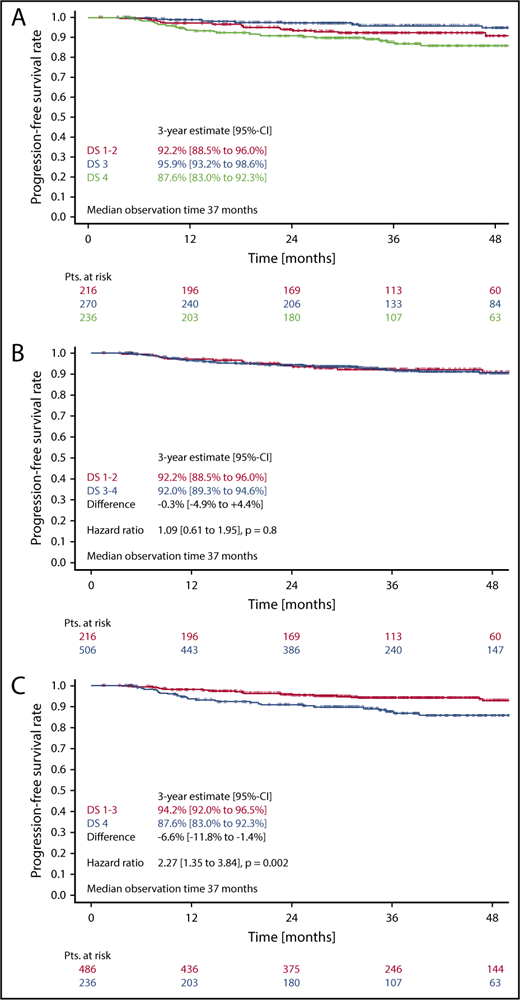
In the survival analysis set of 722 patients receiving 6 cycles of eBEACOPP, 216 had DS1 or DS2, 270 had a DS3, and 236 had a DS4 (note that frequencies in this subset are not representative for the cohort due to the PET-stratified study design). The respective 3-year PFS rates were 92.2% (DS1-2), 95.9% (DS3), and 87.6% (DS4) (Figure 3A). The difference of DS1-2 as compared with DS3 was not statistically significant, with a hazard ratio (HR) of 0.56 (95% confidence interval [CI], 0.26-1.20; P = .14). When taking the mediastinum as cutoff for PET-2 positivity (DS1-2 vs DS3-4), no difference between PET+ and PET− cohorts could be observed concerning PFS (HR, 1.1; 95% CI, 0.6-1.9; P = .8) (Figure 3B). However, the difference in PFS was significant when only DS4 (DS1-3 vs DS4) was considered positive (HR, 2.3; 95% CI, 1.3-3.8; P = .002) (Figure 3C). Multivariate Cox regression models including large mediastinal mass, extranodal involvement, and high IPS confirmed the significant results for the liver as cutoff (DS4) and indicate a prognostic impact of PET-2 beyond the associated baseline risk factors (HR, 2.4; 95% CI, 1.4-4.1; P = .002) (Table 2).
PFS. (A-C) PFS according to DS after 2 cycles of eBEACOPP in patients (Pts.) treated with a total of 6 cycles of eBEACOPP.
PFS. (A-C) PFS according to DS after 2 cycles of eBEACOPP in patients (Pts.) treated with a total of 6 cycles of eBEACOPP.
OS
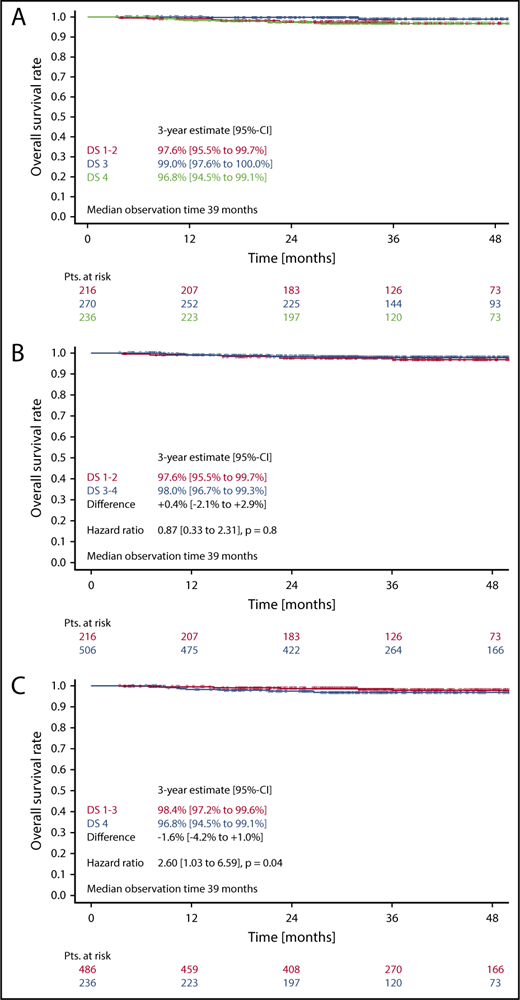
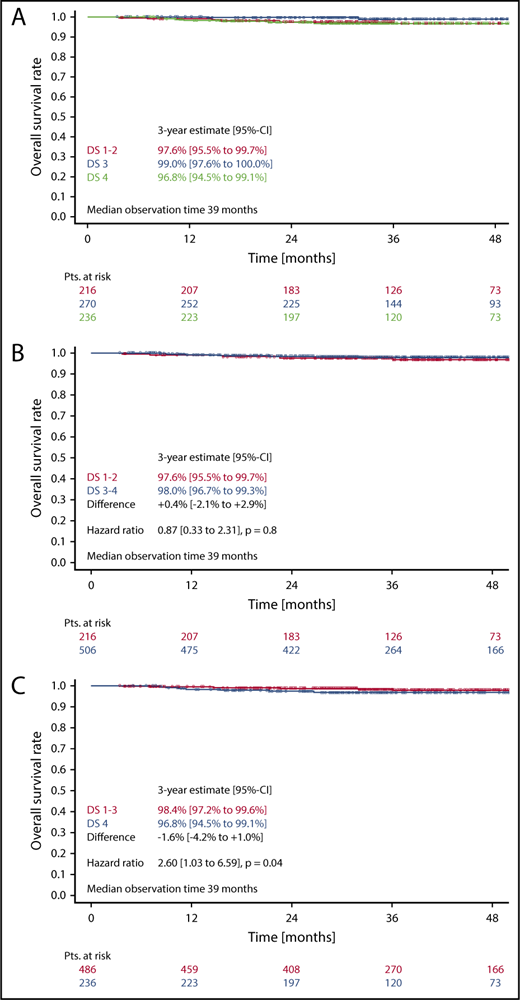
Three-year OS in patients with DS1 or DS2 was 97.6%, as compared with 99.0% in patients with a DS3, and 96.8% in patients with a DS4 (Figure 4A). Here again, the difference between DS1-2 as compared with DS3 only was not statistically significant (HR, 0.27 [0.05-1.34]; P = .11). No difference concerning OS could be observed, when taking the mediastinum as cutoff for PET positivity (DS1-2 vs DS3-4) (HR, 0.9; 95% CI, 0.3-2.3; P = .8) (Figure 4B). In contrast, considering only a residual uptake higher than that in the liver (DS1-3 vs DS4) as positive led to significant OS differences in the univariate (HR, 2.6; 95% CI, 1.0-6.6; P = .04) (Figure 4C) as well as in the multivariate analysis (HR, 3.2; 95% CI, 1.3-8.4; P = .02) (Table 2).
OS. (A-C) OS according to DS after 2 cycles of eBEACOPP in patients treated with a total of 6 cycles of eBEACOPP.
OS. (A-C) OS according to DS after 2 cycles of eBEACOPP in patients treated with a total of 6 cycles of eBEACOPP.
Discussion
Two major findings have emerged from our analyses focusing on the prognostic impact of PET-2 in the HD18 study. First, FDG uptake higher than in the liver after 2 cycles of eBEACOPP (ie, PET-2 DS4) is a highly significant prognostic factor for PFS and OS in multivariate analyses including established baseline risk factors from the IPS, whereas PET-2 DS3 has no prognostic impact and should be rated as negative. Second, even though PET-2 DS4 indicates a significantly higher risk for treatment failure and death, both PFS and OS in this subgroup are still very good with 6 cycles of eBEACOPP and consolidation radiotherapy to PET+ residual disease.
Our findings are in line with the prognostic impact of PET-2 reported for patients with newly diagnosed advanced-stage HL treated with upfront ABVD. Approximately 30% of patients with advanced-stage HL will relapse after completion of ABVD therapy.18-21 Accordingly, PET-2 has been used to select patients at high risk early during treatment in order to increase treatment intensity for them. Three larger studies have been conducted and published so far. All of them used DS4 at PET-2 as cutoff value for PET positivity.10-12 Press et al10 reported 2-year PFS of 64% in patients with DS4 and DS5 with intensified treatment, as compared with 80% to 84% for PET-2− patients following standard ABVD treatment. Gallamini et al11 reported very similar results. Three-year PFS in PET-2− patients was 87% as compared with 57% to 63% after escalation to eBEACOPP with or without rituximab. Johnson et al12 observed a 3-years PFS of 85.7% when treatment was continued with ABVD after negative PET-2. PET-2+ patients who underwent therapy intensification with BEACOPP variants plus or minus radiotherapy had a 3-year PFS of 67.5%. With regard to the unexpected high rate of treatment failures for PET-2− patients in all of these trials (expected, 5%2 ; observed, ∼15%), retrospective analyses were undertaken to define the prognostic impact of the different PET-2 results according to DS. However, these analyses could not find any significant differences between DS1-3. It remains an unanswered question, if the negative predictive value of PET-2 DS1-3 is suited to guide treatment in clinical routine in the context of upfront ABVD therapy. Fortunately, the failure rate of upfront eBEACOPP-treated patients with a negative PET-2 in HD18 is rather low with only 4.7% at 3 years.14 Although PET-2 DS4 is a highly significant prognostic factor in our analysis, these patients still have a 3-year PFS of 92% when treated with 6 cycles of eBEACOPP. This compares obviously favorably to the outcome of PET-2+ patients after initial treatment with ABVD, which is below 70% even when treatment is switched to eBEACOPP. Although conclusions from indirect comparisons between studies must be drawn carefully, these large differences obviously indicate that the prognostic impact of PET-2 is depending on the initial therapy.
Using the FDG uptake of the liver as reference might have additional advantages. First interobserver analyses of the DS had brought promising results with a high level of concordance in response assessment.16 Concordance for the binary decision of PET positivity among 5 reviewers with different levels of experience was improved by rating DS1-3 as PET−.22 Comparable results were obtained by Barrington et al who observed that agreement between different experts and between central reviewers and local readers could be improved by taking the liver as cutoff for PET positivity instead of the mediastinum.23 Thus, the use of PET-2 DS4 as cutoff value should be easier to implement into clinical routine.
The application of this new cutoff value for PET-2 positivity has significant implications for the patients. Within the HD18 study, 48% of all patients had PET-2+ (ie, DS3-4) disease. This percentage comes down to only 24% using DS4 as cutoff value. Consequently, 76% instead of only 52% of all patients can be treated with only 4 cycles of eBEACOPP, following the PET-guided reduction strategy introduced by the HD18 trial. As we did not evaluate the efficacy of this very short and safe treatment approach in patients with DS3 in the HD18 study, this could put those patients at risk for treatment failure. However, the lack of any prognostic impact of PET-2 DS3 in our analysis seems to be robust and reliable. The HD18 study has enrolled >2000 patients and we were able to analyze 722 patients treated prospectively and uniformly with 6 cycles of eBEACOPP. We thus have had the chance to analyze a large and well-documented data set, which we think ameliorates the shortcomings of a retrospective analysis. In addition, we know from many trials that ∼70% of all patients with newly diagnosed advanced-stage HL can be cured with ABVD-based first-line therapy.19 In contrast, ∼30% are high-risk patients, who obviously would benefit from a more intensive regimen like eBEACOPP. With the use of PET-2 DS4 as cutoff value, we select a number of patients (24%), which corresponds to this clinical experience. We thus feel safe to recommend using this cutoff value in clinical routine. However, patients who are treated with a total of only 4 cycles of eBEACOPP, owing to DS3 after 2 cycles, should be followed carefully both inside and outside of clinical trials, as this practice is mainly based on indirect evidence.
In conclusion, only residual uptake above that in the liver at PET-2 (ie, DS4) is an important risk factor regarding survival outcomes for patients treated with eBEACOPP upfront. We thus recommend DS4 as cutoff value for PET-2 positivity. However, even high-risk patients (DS4) have a favorable outcome when treated with 6 cycles of eBEACOPP followed by radiotherapy to PET+ residual disease. Importantly, this cutoff score allows treatment to be reduced to only 4 cycles of eBEACOPP in ∼75% of patients, and results in both excellent tolerability and efficacy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Participation in the HD18 trial implicated potential loss of efficacy for patients with a negative PET-2 randomized to receive only 4 cycles of eBEACOPP. The authors thank all participating patients, their families, and their treating physicians for accepting this potentially increased risk of treatment failure and thereby helping to improve the treatment of future HL patients. The authors thank all participating PET centers for their continuous support.
The HD18 trial was supported by the Deutsche Krebshilfe (no. 107957 and 110617) and the Swiss State Secretariat for Education, Research and Innovation (SERI), and by Roche Pharma AG (no. ML-21683).
Authorship
Contribution: C.K., H.G., and P.B. designed the present analysis; H.G. led the statistical analyses of the data; A.E. was the principal investigator for the study; all authors contributed to data interpretation, reviewed the draft, and approved the final version of this report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carsten Kobe, Department of Nuclear Medicine, University Hospital of Cologne, Kerpener Str. 62, 50937 Cologne, Germany; e-mail: carsten.kobe@uk-koeln.de.