Key Points
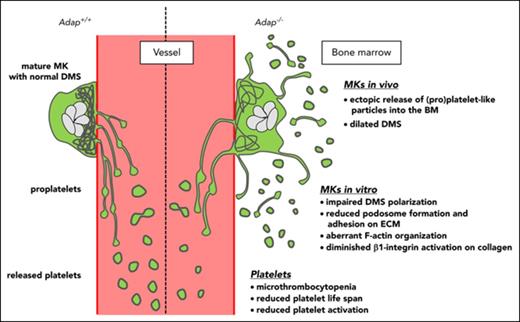
ADAP deficiency in mice leads to microthrombocytopenia caused by a reduced platelet life span and ectopic (pro)platelet release.
Lack of ADAP in MKs impairs demarcation membrane system polarization and podosome formation.
Abstract
Bone marrow (BM) megakaryocytes (MKs) produce platelets by extending proplatelets into sinusoidal blood vessels. Defects in thrombopoiesis can lead to thrombocytopenia associated with increased bleeding tendency. Recently, the platelet disorder congenital autosomal-recessive small-platelet thrombocytopenia (CARST) was described; it is caused by mutations in the adhesion and degranulation-promoting adaptor protein (ADAP; synonym: FYB, SLAP130/120) gene, and characterized by microthrombocytopenia and bleeding symptoms. In this study, we used constitutive ADAP-deficient mice (Adap−/−) as a model to investigate mechanisms underlying the microthrombocytopenia in CARST. We show that Adap−/− mice display several characteristics of human CARST, with moderate thrombocytopenia and smaller-sized platelets. Adap−/− platelets had a shorter life span than control platelets, and macrophage depletion, but not splenectomy, increased platelet counts in mutant mice to control levels. Whole-sternum 3-dimensional confocal imaging and intravital 2-photon microscopy revealed altered morphology of ADAP-deficient MKs with signs of fragmentation and ectopic release of (pro)platelet-like particles into the BM compartment. In addition, cultured BM-derived MKs lacking ADAP showed reduced spreading on extracellular matrix proteins as well as activation of β1 integrins, impaired podosome formation, and displayed defective polarization of the demarcation membrane system in vitro. MK-/platelet-specific ADAP-deficient mice (PF4-cre) also produced fewer and smaller-sized platelets and released platelets ectopically. These data demonstrate that the abnormal platelet production in the mutant mice is an MK-intrinsic defect. Taken together, these results point to an as-yet-unidentified role of ADAP in the process of MK polarization and platelet biogenesis.
Introduction
Platelets are small anucleate cell fragments that are continuously produced from bone marrow (BM) megakaryocytes (MKs),1 and very recently, also the lung was suggested to be a site of platelet production.2 MKs are adjacent to sinusoidal blood vessels, where they extend long cytoplasmic protrusions, called proplatelets, into BM sinusoids, from which platelets are sequentially released.3 However, the molecular mechanisms orchestrating platelet biogenesis are incompletely understood, and defects in thrombopoiesis can cause thrombocytopenia, which is a major clinical problem. Reorganization of the MK cytoskeleton is critical to platelet production,4,5 as it was shown that dynein-driven microtubule sliding is the primary driving force of proplatelet extension,6,7 whereas the F-actin network is important for the maturation and positioning of the demarcation membrane system (DMS),8,9 the initiation of proplatelet formation10 and proplatelet branching.4
Congenital autosomal-recessive small-platelet thrombocytopenia (CARST) was recently described as a platelet disorder with a low platelet count and a decreased mean platelet volume (MPV). So far, 8 patients in 2 families have been described suffering from mild bleeding episodes to heavy menstrual bleedings.11-13 None of the patients had growth defects, immune defects, or unusual infections.11-13 This is in contrast to microthrombocytopenic patients with Wiskott-Aldrich syndrome or patients carrying mutations in the ARPC1B gene, which are characterized, among others, by immunodeficiency.14,15 Genomic analyses revealed that mutations in the adhesion and degranulation-promoting adaptor protein (ADAP; synonyms: FYB, SLAP-130/120) gene are the underlying cause for CARST in humans.11,12 Two mutations have been reported (point mutation 393G>A: W131X; 2-bp deletion 1385_1386delAT: Y462) leading to a frameshift and the insertion of a premature stop codon, presumably resulting in a truncated gene product and no functional ADAP protein.11,12 ADAP is primarily expressed in hematopoietic cells, but not in B cells (reviewed in Wang and Rudd16 ). The ADAP protein contains a proline-rich region and an Src homology 3 (SH3)-like domain (which bind to Skap55), phosphotyrosine sites (that bind the SH2 domains of Fyn and Slp-76), 2 putative nuclear-localization sites, and an enabled/vasodilator-stimulated phosphoprotein (Ena/VASP) homology 1 domain (which binds to Ena/VASP proteins).17 Several studies have investigated the role of ADAP in platelet function. It was shown that ADAP is involved in von Willebrand factor/glycoprotein Ib-IX-V (GP-Ib-IX-V) signaling (leading to αIIbβ3 activation in vitro), promotes fibrinogen binding to αIIbβ3 (through interaction with talin and kindlin-3), and is an essential component of integrin-mediated mechanotransduction (thereby promoting F-actin assembly and thrombus stabilization).18-20 Another group demonstrated that ADAP plays a role in mediating platelet activation via the collagen-binding integrin α2β1.21
However, only little is known about the role of ADAP in platelet biogenesis. To investigate this, we capitalized on constitutive as well as conditional ADAP knockout mice and demonstrate for the first time that ADAP deficiency in mice leads to a microthrombocytopenia as observed in CARST patients, caused by a reduced platelet life span and the ectopic release of (pro)platelet-like particles into the BM compartment. ADAP-null MKs showed a DMS polarization defect, reduced actin-rich podosome formation, and impaired β1 integrin–collagen interaction. These findings establish a novel role for ADAP in platelet biogenesis.
Materials and methods
Mice
Constitutive ADAP-deficient mice (Adap−/−) were generated as described previously.22 Conditional ADAP-deficient mice were generated by intercrossing Adapfl/fl (HEPD0569_8_D07 EUCOMM) mice (exon 2 flanked by loxP sites) with mice carrying the Cre-recombinase under the platelet factor 4 (PF4) promoter.23 All mice used in this study were 6 to 14 weeks old. All animal experiments were approved by the district government of Lower Frankonia (Bezirksregierung Unterfranken).
All methods and the statistical analysis are described in supplemental Material and methods (available on the Blood Web site).
Results
ADAP-deficient mice display a microthrombocytopenia
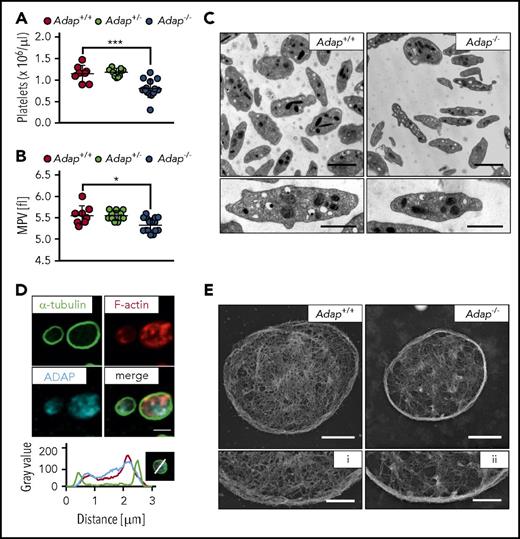
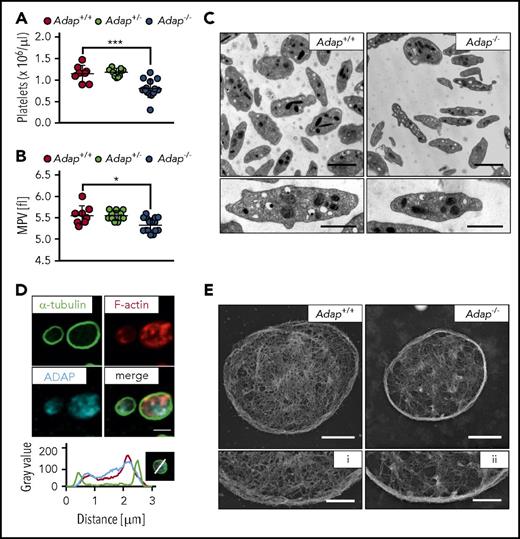
To investigate the function of ADAP in platelet biogenesis, we capitalized on constitutive ADAP knockout mice and confirmed lack of the ADAP protein in platelet lysates of Adap−/− mice by western blot analysis, whereas the protein was detected with an apparent molecular weight of about 130 kDa in controls (supplemental Figure 1). Mice lacking ADAP displayed a significant reduction in platelet count as determined by a hematology analyzer (Figure 1A), and a small but significant reduction in MPV (Figure 1B), whereas heterozygous mice displayed platelet counts and size comparable to controls. Other blood cell parameters were only slightly changed in ADAP-deficient mice (supplemental Table 1). Next, we investigated whether the knockout of ADAP leads to ultrastructural changes in platelets. Transmission electron microscopic (TEM) images of knockout platelets showed an overall normal morphology, with comparable density of granules (Figure 1C; supplemental Figure 2A). TEM analysis also confirmed reduced platelet size as revealed by determination of platelet area (supplemental Figure 2B-C) with decreased length and width axes of the discoid mutant platelets (supplemental Figure 2D-E). Thus, Adap−/− mice recapitulate the microthrombocytopenia observed in CARST patients.
Microthrombocytopenia in ADAP-deficient mice. (A) Platelet count per microliter and (B) MPV were determined from Adap−/−, Adap+/− and control mice by a hematology analyzer. (A-B) Values are mean ± standard deviation (SD; n = at least 8). ***P < .001; *P < .05. (C) Transmission electron micrographs from Adap+/+ and Adap−/− platelets; scale bars represent 2 µm (top panel) and 1 µm (bottom panel). (D) ADAP localization in resting control platelets was assessed by confocal microcopy; scale bar represents 2 µm. Line plot histogram of the marked platelet in the bottom right corner. (E) Images of the platelet cytoskeleton ultrastructure on poly-l-lysine. Scale bars represent 1 µm (top panel) and 0.5 µm (bottom panel; i, ii).
Microthrombocytopenia in ADAP-deficient mice. (A) Platelet count per microliter and (B) MPV were determined from Adap−/−, Adap+/− and control mice by a hematology analyzer. (A-B) Values are mean ± standard deviation (SD; n = at least 8). ***P < .001; *P < .05. (C) Transmission electron micrographs from Adap+/+ and Adap−/− platelets; scale bars represent 2 µm (top panel) and 1 µm (bottom panel). (D) ADAP localization in resting control platelets was assessed by confocal microcopy; scale bar represents 2 µm. Line plot histogram of the marked platelet in the bottom right corner. (E) Images of the platelet cytoskeleton ultrastructure on poly-l-lysine. Scale bars represent 1 µm (top panel) and 0.5 µm (bottom panel; i, ii).
Size and shape of cells are mainly determined by the cytoskeleton and we could recently show that increased microtubule stability leads to microtubule bending and thereby probably contributes to a decrease in platelet size.24 Here, TEM analysis revealed a similar number of microtubules in ADAP-deficient and control platelets (supplemental Figure 2F). In addition, Adap−/− platelets could disassemble and reassemble their microtubules with no change in platelet size after microtubule reassembly, indicating that ADAP is dispensable for microtubule stability (supplemental Figure 3A-C). Because ADAP has been described to interact with actin-regulatory proteins (reviewed in Wang and Rudd16 ), we investigated the localization of ADAP in resting platelets and found that it is rather associated with the F-actin network than with microtubules (Figure 1D), confirming previous data.20 We analyzed the cytoskeletal ultrastructure of ADAP-deficient platelets by electron microscopy and observed a partly incomplete and fragmented F-actin network (Figure 1E). F-actin assembly after platelet activation with a low dose of thrombin was slightly decreased in Adap−/− platelets (supplemental Figure 3D). These data suggest that loss of ADAP mildly affects actin organization in platelets. It is tempting to speculate that the altered F-actin network could account for the smaller platelet size. However, further studies are required to test this hypothesis.
Moderately reduced activation and shorter life span of ADAP-deficient platelets
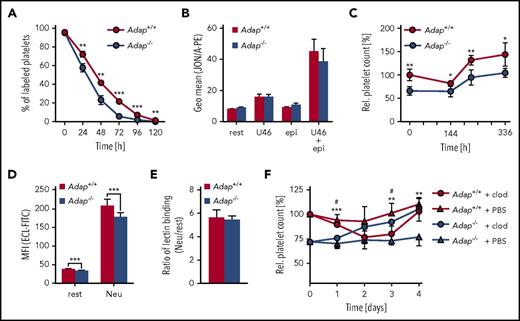
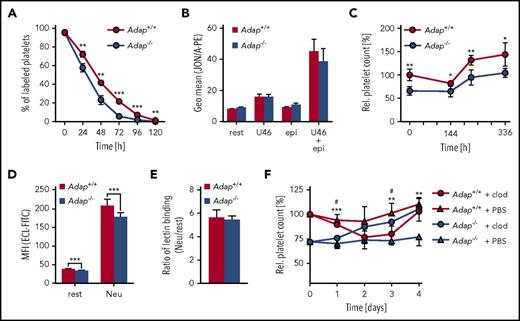
Analysis of surface expression of major platelet glycoproteins revealed a significant reduction of GPIb, GPV, GPIX, CD9, and integrin α2 and β1 abundance on Adap−/− platelets (supplemental Figure 4A), probably a consequence of the smaller size of the cells. ADAP-deficient platelets exhibited moderately reduced β1 and αIIbβ3 integrin activation as well as diminished P-selectin exposure upon stimulation with classic agonists (supplemental Figure 4B-C). To test whether an accelerated platelet turnover is a major cause of thrombocytopenia in ADAP-deficient mice, we determined the platelet life span and found a small, but significant, reduction (Figure 2A; Adap+/+, t1/2: 41.3 ± 2.3 hours; Adap−/−, t1/2: 29.3 ± 2.9 hours; P < .001), indicating an increased clearance of the mutant cells. Flow cytometric analysis showed that Adap−/− platelets were not preactivated by measuring αIIbβ3 activation under resting conditions and after stimulation with epinephrine, adenosine 5′-diphosphate, or the thromboxane analog U46619 (Figure 2B; supplemental Figure 4C). The spleen can function as the site of platelet sequestration and subsequent removal. However, we observed no splenomegaly and only a moderate increase in MK number in spleens of mutant mice (supplemental Figure 5). Of note, splenectomy in control and knockout mice had no long-term effects on platelet counts (Figure 2C). The platelet life span is also determined by the continuous loss of sialic acids from glycoproteins that triggers platelet removal by interaction of exposed galactose with the hepatic Ashwell-Morell receptor.25 Platelets from Adap−/− mice had a small, but significant, decrease in Erythrina cristagalli lectin (ECL) binding to exposed galactose (Figure 2D). However, the ratio of platelets treated with neuraminidase (100% desialylation) vs untreated ones was comparable between control and knockout samples (Figure 2E), indicating no major alteration in terminal galactose levels. Of note, the proportion of circulating young, reticulated platelets in blood samples of ADAP-deficient mice stained with thiazol orange was not increased but rather decreased, strongly suggesting that the increased clearing occurred independent of platelet age (supplemental Figure 6A). Interestingly, macrophage depletion resulted in a continuous increase of the platelet count in ADAP-deficient mice to almost control levels after 4 days (Figure 2F). These data suggest that the increased clearance of platelets in ADAP-deficient mice is mainly triggered by macrophages, contributing to the observed thrombocytopenia. Furthermore, we tested whether increased platelet turnover predominantly affects smaller platelets. However, we did not observe this in ADAP-deficient mice (data not shown) indicating that altered platelet size is not the triggering event for the increased platelet clearance. Of note, ADAP-deficient platelets displayed an increased tendency of Ca2+ stress-induced phosphatidylserine exposure during storage in vitro (supplemental Figure 6B), which may in part explain the enhanced clearance of Adap−/− platelets by macrophages.
Reduced platelet life span in Adap−/−mice. (A) Platelet life span was analyzed by injection of a Dylight488 anti−GPIX antibody and the fluorescently labeled platelet population was measured over 5 days by flow cytometry. (B) αIIbβ3 integrin activation was analyzed by JON/A-PE binding under resting (rest) conditions and upon stimulation with 1 µM U46619 (U46; thromboxane analog), 10 µM epinephrine (epi) alone and in combination. Relative platelet count was determined after (C) splenectomy or (F) macrophage depletion by flow cytometry. *Significance between the control groups; #significance between the macrophage depletion groups. (D) Desialylation of Adap+/+ and Adap−/− platelets in washed blood was determined with fluorescein isothiocyanate (FITC)-labeled ECL binding by flow cytometry. Platelets were treated with neuraminidase (Neu) or untreated (rest). (E) Ratio of lectin binding between neuraminidase treated and untreated samples. (A-F) Values are mean ± SD (at least n = 5). ***P < .001; **P < .01; */#P < .05.
Reduced platelet life span in Adap−/−mice. (A) Platelet life span was analyzed by injection of a Dylight488 anti−GPIX antibody and the fluorescently labeled platelet population was measured over 5 days by flow cytometry. (B) αIIbβ3 integrin activation was analyzed by JON/A-PE binding under resting (rest) conditions and upon stimulation with 1 µM U46619 (U46; thromboxane analog), 10 µM epinephrine (epi) alone and in combination. Relative platelet count was determined after (C) splenectomy or (F) macrophage depletion by flow cytometry. *Significance between the control groups; #significance between the macrophage depletion groups. (D) Desialylation of Adap+/+ and Adap−/− platelets in washed blood was determined with fluorescein isothiocyanate (FITC)-labeled ECL binding by flow cytometry. Platelets were treated with neuraminidase (Neu) or untreated (rest). (E) Ratio of lectin binding between neuraminidase treated and untreated samples. (A-F) Values are mean ± SD (at least n = 5). ***P < .001; **P < .01; */#P < .05.
Ectopic release of (pro)platelet-like particles into the BM compartment in Adap−/− mice
We next investigated whether a platelet production defect accounted for the thrombocytopenia in Adap−/− mice in addition to the enhanced clearance in vivo. We first used a model of antibody-mediated platelet depletion to synchronize platelet production, thereby increasing the population of young platelets in the circulation. When we determined the kinetics of platelet recovery after depletion, we could not detect any differences between ADAP-deficient and control mice under these conditions (supplemental Figure 6C). These results suggest that ADAP is required for normal platelet production at steady state in vivo but not under conditions of acute elevated platelet demand.
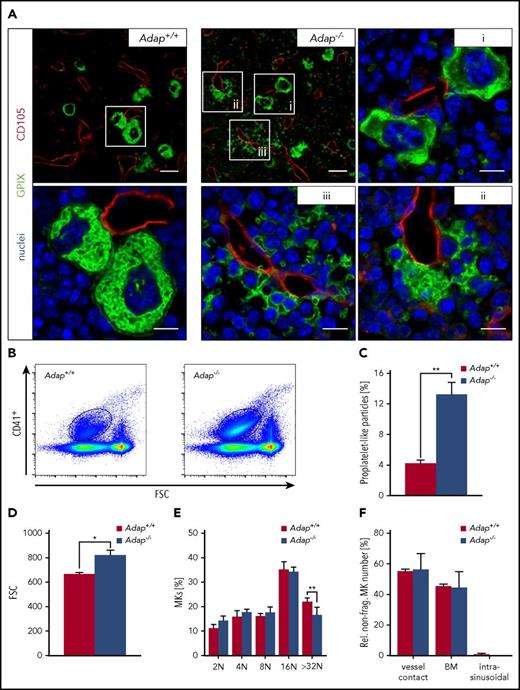
Strikingly, we observed besides morphological normal MKs, a massive accumulation of GPIX+ particles and also fragmented MKs in the BM compartment in immunostained BM cross-section of intact femora from ADAP-deficient mice (Figure 3A). Quantitative analysis of BM samples by flow cytometry revealed a threefold increase of αIIbβ3+ particles in mutant mice compared with controls, strongly suggesting that these fragments originated from MKs (Figure 3B-C). Interestingly, the size of these particles was increased in ADAP-deficient BM suggesting the presence of pre- and/or proplatelets (Figure 3D). We further analyzed whether these fragments contain a marginal band, a hallmark feature of platelets. Interestingly, we found in the BM of mutant mice fragments with a tubulin ring comparable to platelets in the bloodstream (supplemental Figure 7A), but also fragments with a diffuse tubulin staining, a phenomenon also observed by others.26 Together, these data indicate that a mixture of proplatelets, preplatelets, and platelets are ectopically released. Ploidy levels of mutant BM MKs were unaltered to controls with only a slight decrease in >32 N MKs, possibly due to fragmentation of mature Adap−/− MKs (Figure 3E). Number of nonfragmented MKs (Adap+/+ 10.95 ± 0.82 MKs per visual field vs Adap−/− 9.52 ± 0.58 MKs per visual field; 387.5 µm × 387.5 µm; P > .05) as well as the localization of those MKs within the BM was unaltered (Figure 3F). Expression analysis of major glycoproteins on BM MKs from ADAP-deficient mice by flow cytometry revealed an overall normal expression with only slightly reduced αIIbβ3 expression (supplemental Figure 8). To test, whether loss of ADAP generally affects proplatelet formation, we analyzed the capacity of fetal liver cell (FLC)-derived MKs to form proplatelets in vitro (supplemental Figure 9A). Under these conditions, we observed normal proplatelet formation of ADAP-deficient FLC-derived MKs, suggesting that the MK phenotype observed in the BM from the deficient mice was not due to a general proplatelet formation defect (supplemental Figure 9B). Interestingly, the area of the marginal tubulin band from single Adap−/− platelets released in culture was decreased (supplemental Figure 9C), which is in line with the observed smaller platelet size in the peripheral blood (Figure 1B), and suggests an MK-intrinsic defect.
Release of (pro)platelet-like particles into the BM compartment of Adap−/−mice. (A) Representative confocal microscopy images of immunostained cryosections from femura of control and ADAP-deficient mice. Sinusoides (red, CD105), MKs (green, GPIX), and nuclei (blue, DAPI). Scale bars represent 30 µm and 10 µm (insets). (B-D) Gating strategy and analysis of (pro)platelet-like particles (CD41+) in the BM by flow cytometry. Values are mean ± SD (n = 3). **P < .01; *P < .05. (E) Ploidy level of BM MKs was assessed by flow cytometry. Values are mean ± SD (n = 6; **P < .01). (F) Analysis of localization of nonfragmented MKs in the BM based on cryosections. Three categories were defined: vessel contact (MKs with contact to sinusoids); BM (MKs in the BM compartment with no contact to sinusoids) and intrasinusoidal (MKs inside the sinusoid lumen). Values are mean ± SD. Three cryosections per femura from each genotype (3 vs 3 mice) and at least 5 images per cryosections were analyzed.
Release of (pro)platelet-like particles into the BM compartment of Adap−/−mice. (A) Representative confocal microscopy images of immunostained cryosections from femura of control and ADAP-deficient mice. Sinusoides (red, CD105), MKs (green, GPIX), and nuclei (blue, DAPI). Scale bars represent 30 µm and 10 µm (insets). (B-D) Gating strategy and analysis of (pro)platelet-like particles (CD41+) in the BM by flow cytometry. Values are mean ± SD (n = 3). **P < .01; *P < .05. (E) Ploidy level of BM MKs was assessed by flow cytometry. Values are mean ± SD (n = 6; **P < .01). (F) Analysis of localization of nonfragmented MKs in the BM based on cryosections. Three categories were defined: vessel contact (MKs with contact to sinusoids); BM (MKs in the BM compartment with no contact to sinusoids) and intrasinusoidal (MKs inside the sinusoid lumen). Values are mean ± SD. Three cryosections per femura from each genotype (3 vs 3 mice) and at least 5 images per cryosections were analyzed.
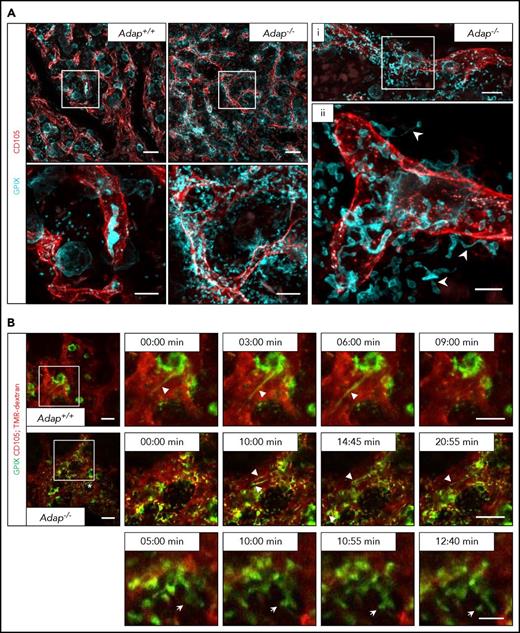
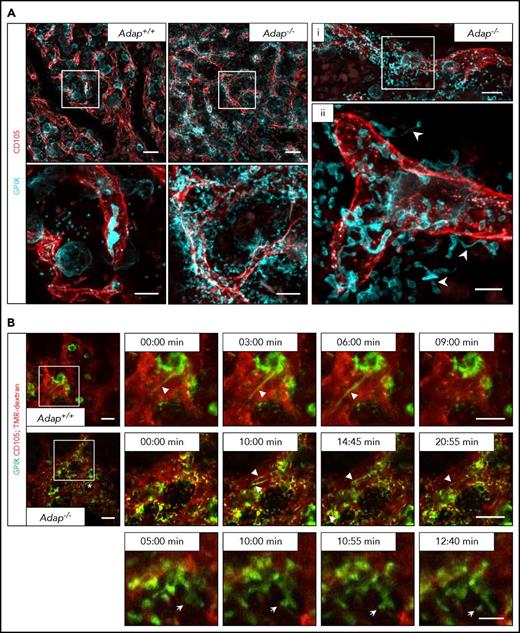
Next, we investigated the localization of (pro)platelet-like particles in the BM compartment in Adap−/− mice in more detail. Therefore, we performed in vivo staining of vessels and MKs/platelets, perfusion fixation, and subsequent clearing of the sternum from ADAP-deficient and control mice (Figure 4A; supplemental Videos 1-4). Maximum projection of z-stacks of transparent sterna obtained by confocal microscopy from Adap−/− mice revealed the presence of MKs next to sinusoids with protrusions extending into the BM compartment, proplatelets (Figure 4A; supplemental Videos 2-3), and platelets (supplemental Figure 7B; supplemental Video 4) residing next to the vessel. These data further suggest defects in producing proplatelets into the vessel lumen due to disorientation of MK extensions. Intravital 2-photon microscopy imaging not only confirmed the release of (pro)platelet-like particles into the BM compartment but also showed that ADAP-deficient MKs are in principle able to form proplatelets into the vessel lumen in vivo (Figure 4B; supplemental Videos 5-8). These data clearly demonstrate that reduced platelet life span and the ectopic release of (pro)platelet-like particles into the BM compartment to a great extent account for the observed thrombocytopenia, and suggest that ADAP plays a critical role in the final stages of platelet production in vivo.
Release of (pro)platelet-like particles next to the vessels in ADAP-deficient mice. (A) Maximum projection of 60 µm z-stacks obtained by confocal microscopy from sternal BM of control and ADAP-deficient mice. Vessels (red; CD105), MKs (cyan; GPIX). Sternum was fixed and cleared for imaging. Scale bars represent 50 µm (top panel; left) and 20 µm (bottom panel; left). Bottom panel, Digital magnification of the region marked in the top panel. (i, ii) Maximum projection of a 25 µm z-stack from a sternum of an ADAP-deficient mouse. Scale bars represent 30 µm (i) and 10 µm (ii). Arrowheads in (ii) point to proplatelets released into the BM compartment. (B) Intravital 2-photon microscopy of proplatelet formation in the skull at the indicated time points. Vessels were visualized by staining of CD105 and with tetramethylrhodamine-dextran (TMR-dextran; red); MKs/platelets are shown by GPIX staining (green). Scale bars represent 50 µm (top and middle panel) and 10 µm (bottom panel). Arrowheads point to proplatelets (top and middle panel), arrows point to platelet-like particle released into the BM compartment (bottom panel) in the region marked with an asterisk (middle panel, left).
Release of (pro)platelet-like particles next to the vessels in ADAP-deficient mice. (A) Maximum projection of 60 µm z-stacks obtained by confocal microscopy from sternal BM of control and ADAP-deficient mice. Vessels (red; CD105), MKs (cyan; GPIX). Sternum was fixed and cleared for imaging. Scale bars represent 50 µm (top panel; left) and 20 µm (bottom panel; left). Bottom panel, Digital magnification of the region marked in the top panel. (i, ii) Maximum projection of a 25 µm z-stack from a sternum of an ADAP-deficient mouse. Scale bars represent 30 µm (i) and 10 µm (ii). Arrowheads in (ii) point to proplatelets released into the BM compartment. (B) Intravital 2-photon microscopy of proplatelet formation in the skull at the indicated time points. Vessels were visualized by staining of CD105 and with tetramethylrhodamine-dextran (TMR-dextran; red); MKs/platelets are shown by GPIX staining (green). Scale bars represent 50 µm (top and middle panel) and 10 µm (bottom panel). Arrowheads point to proplatelets (top and middle panel), arrows point to platelet-like particle released into the BM compartment (bottom panel) in the region marked with an asterisk (middle panel, left).
Lack of ADAP impairs MK DMS polarization in vitro
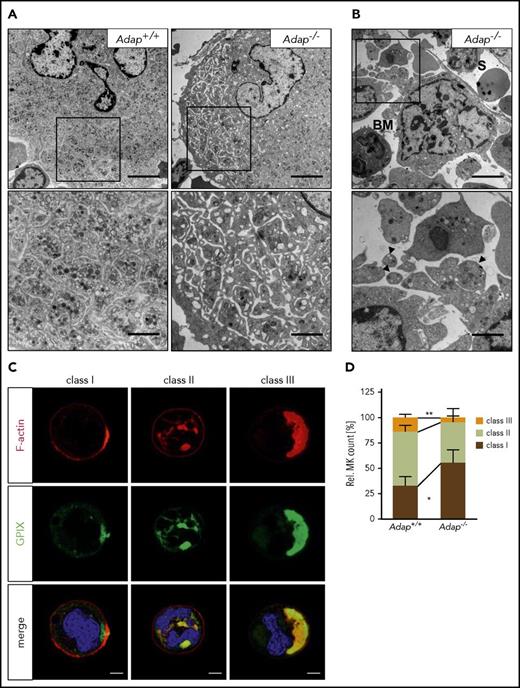
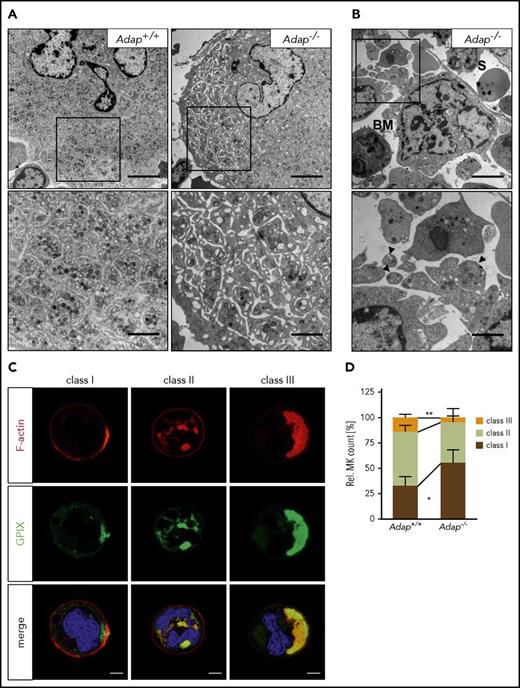
The ectopic release of (pro)platelet-like structures prompted us to examine the ultrastructure of ADAP-deficient BM MKs. MKs lacking ADAP formed a normal DMS but also at times we observed MKs with dilated DMS, suggesting an abnormal anchorage of membranes to the cytoskeleton (Figure 5A; supplemental Figure 10). In addition, we found fragmented MKs close to sinusoids with ectopically released (pro)platelet-like particles (Figure 5B; supplemental Figure 10), confirming our results obtained by immunostaining. These observations point to a nonhomogeneous maturation of the DMS or a defect in coordination of the cytoskeleton forces that drive DMS organization.
Defective DMS polarization of ADAP-deficient MKs in vitro. (A-B) Transmission electron micrographs from intact BM of control and ADAP-deficient mice. Scale bars represent 5 µm (top panel) and 2 µm (bottom panel, inset). (B) Release of (pro)platelet-like particles into the BM compartment (BM) next to a sinusoid (S) (top panel). Higher magnification of released (pro)platelet-like particles (arrowheads; bottom panel). (C) Representative confocal images of Adap+/+ MKs cultured in vitro for 5 days and stained for F-actin (red), GPIX (green), and the nucleus (blue). According to the localization of GPIX and F-actin as well as the position of the nucleus 3 classes were defined (class I-III). Scale bar represents 10 µm. (D) Quantitative analysis of the distribution of the 3 MK classes in vitro on day 5 of cultivation. Five hundred MKs per genotype were analyzed. Values are mean ± SD (n = 5) (**P < .01; *P < .05).
Defective DMS polarization of ADAP-deficient MKs in vitro. (A-B) Transmission electron micrographs from intact BM of control and ADAP-deficient mice. Scale bars represent 5 µm (top panel) and 2 µm (bottom panel, inset). (B) Release of (pro)platelet-like particles into the BM compartment (BM) next to a sinusoid (S) (top panel). Higher magnification of released (pro)platelet-like particles (arrowheads; bottom panel). (C) Representative confocal images of Adap+/+ MKs cultured in vitro for 5 days and stained for F-actin (red), GPIX (green), and the nucleus (blue). According to the localization of GPIX and F-actin as well as the position of the nucleus 3 classes were defined (class I-III). Scale bar represents 10 µm. (D) Quantitative analysis of the distribution of the 3 MK classes in vitro on day 5 of cultivation. Five hundred MKs per genotype were analyzed. Values are mean ± SD (n = 5) (**P < .01; *P < .05).
It has been demonstrated that the organization of the DMS relies on F-actin rearrangement to polarize membranes and nuclei within MKs.8,9 Given the role of ADAP for actin arrangement in platelets and coordinated proplatelet formation in the BM, we hypothesized that ADAP may influence DMS polarization. To test this, we cultured BM MKs in vitro and analyzed DMS polarization by confocal microscopy similar to how it was described in Antkowiak et al.8 We classified MKs into different groups depending on the localization of the DMS (GPIX signal) and the nucleus (Figure 5C). MKs with a nonpolarized DMS were grouped in class I and II, with only 1 peripheral signal for GPIX (class I) or more signals also between nuclear lobes (class II), whereas class III MKs displayed a polarized DMS cap on 1 side of the cell separated from the nucleus. Analysis of MK cultures on day 5 revealed a significant reduction of class III and an increase in class I ADAP-deficient MKs (Figure 5D). Impaired polarization was still present when we analyzed cultures on days 6 and 7 (supplemental Figure 11A). Of note, the diameter as well as the ploidy was comparable between cultured Adap+/+ and Adap−/− BM MKs (supplemental Figure 11B-E). Taken together, we conclude that the observed defect in DMS polarization in vitro is rather a consequence of defective cytoskeletal dynamics than defective MK maturation, and that this defect might contribute to the ectopic release of (pro)platelet-like particles into the BM compartment in vivo.
Adap−/− MKs spread on collagen display defective F-actin organization
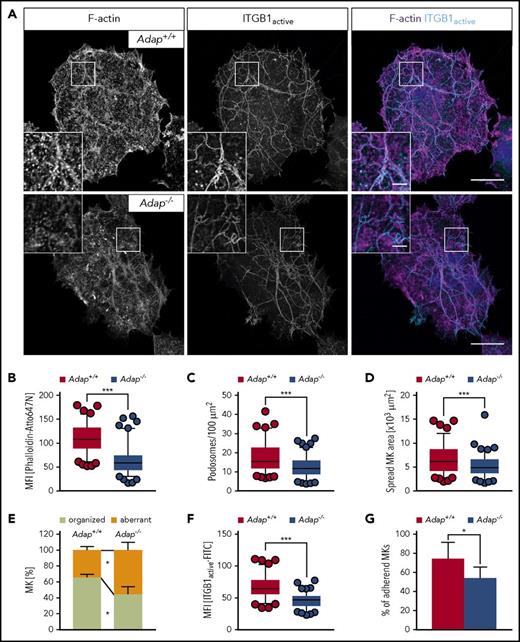
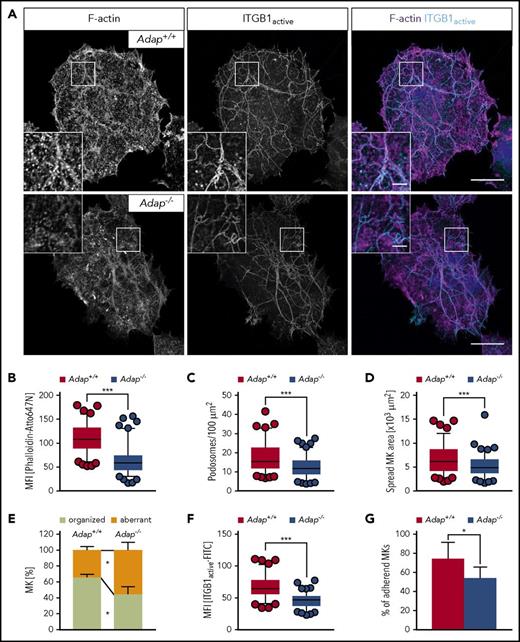
Podosomes have been proposed to be modulators of MK interactions with matrix proteins, and to be important for proplatelet penetration through the basement membrane.27 Podosomes consist of an actin-rich core structure and the surrounding ring is characterized by adhesion plaque proteins such as integrins and multiple adaptor proteins.28 The stability and dynamics of podosomes were shown to be dependent on actin cytoskeletal dynamics.27 Thus, we hypothesized that ADAP could be important for podosome formation, MK adhesion to the extracellular matrix, and, consequently, directional proplatelet formation into the bloodstream. Semeniak et al showed that fibronectin, collagen IV and collagen I are present throughout the BM cavity.29 Collagen I is also found around larger arterioles and arteries, and subjacent to sinusoids, whereas collagen IV is localized to basement membranes of sinusoids.29 At first, a quantitative assessment of MK adhesion and spreading on Horm collagen (native type I fibrils from equine tendon) was performed (Figure 6A). Spread Adap−/− MKs displayed defective actin polymerization as reflected by a strongly decreased mean fluorescent intensity when stained with phalloidin-Atto647N (Figure 6B). This defect translated into reduced podosome density and area of spread MKs (Figure 6C-D). In addition, ∼60% of control MKs displayed an organized F-actin network along collagen fibers, whereas this was only seen in ∼40% of ADAP-deficient MKs (supplemental Figure 12; Figure 6E). Similar defects were also observed for MKs spread on fibronectin and nonfibrillar collagen I, whereas on collagen IV only the spread area of MKs was reduced (supplemental Figure 13). β1 integrins interact with collagen and fibronectin, and since loss of ADAP in platelets impairs integrin activation in platelets (supplemental Figure 4; Kasirer-Friede et al19 ), we investigated whether impaired actin arrangement in MKs causes altered integrin function. Indeed, β1 integrin activation of spread ADAP-deficient MKs was reduced as measured by the mean fluorescence intensity of fluorophore-coupled antibodies against active β1 integrin (Figure 6F), and adhesion of mutant MKs was impaired on Horm collagen (Figure 6G). Collectively, these data show that Adap−/− MKs exhibit defective F-actin organization, resulting in impaired adhesion to the extracellular matrix proteins collagen I and fibronectin, and suggest that ADAP in MKs functions as a linker protein between integrin affinity and F-actin–dependent DMS polarization, and loss of ADAP results in ectopic, nondirectional (pro)platelet release into the BM compartment.
Defective F-actin organization and β1 integrin activation in ADAP-deficient MKs upon spreading on Horm collagen. (A) Representative confocal images of cultured control and ADAP-deficient MKs 3 hours after spreading on Horm collagen, and stained for F-actin and active ITGB1 (β1 integrin). Scale bars represent 30 µm and 5 µm (inset). (B) Mean fluorescence intensity of F-actin (B) and active ITGB1 (F), and density of podosomes (C) in the lowest optical section of spread MKs. Area (D) and F-actin organization along collagen fibers (E) of spread MKs. (A-F) 100 MKs per genotype were analyzed. Box-and-whiskers plots display 5 and 95 percentiles. Data points below and above the whiskers are shown as individual points. The line in the middle of the box is plotted at the median (***P < .001). (G) Relative adhesive MKs in percent after 3 hours; representative graph from 2 independent experiments. Two biological replicates and 3 technical replicates were analyzed per experiments (*P < .05).
Defective F-actin organization and β1 integrin activation in ADAP-deficient MKs upon spreading on Horm collagen. (A) Representative confocal images of cultured control and ADAP-deficient MKs 3 hours after spreading on Horm collagen, and stained for F-actin and active ITGB1 (β1 integrin). Scale bars represent 30 µm and 5 µm (inset). (B) Mean fluorescence intensity of F-actin (B) and active ITGB1 (F), and density of podosomes (C) in the lowest optical section of spread MKs. Area (D) and F-actin organization along collagen fibers (E) of spread MKs. (A-F) 100 MKs per genotype were analyzed. Box-and-whiskers plots display 5 and 95 percentiles. Data points below and above the whiskers are shown as individual points. The line in the middle of the box is plotted at the median (***P < .001). (G) Relative adhesive MKs in percent after 3 hours; representative graph from 2 independent experiments. Two biological replicates and 3 technical replicates were analyzed per experiments (*P < .05).
Discussion
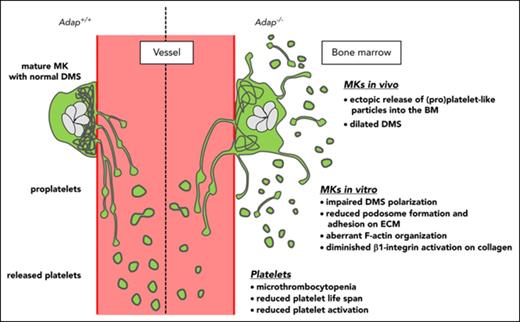
Our study demonstrates for the first time that the hematopoietic adapter protein ADAP plays a crucial role in the terminal steps of platelet production. The major conclusions are: (1) lack of ADAP in mice leads to microthrombocytopenia reproducing key features of the phenotype of CARST patients; (2) the reduced platelet count is caused by ectopic release of (pro)platelets into the BM compartment and a shortened platelet life span; and (3) ADAP is required for DMS polarization, F-actin organization, and integrin activation in spread MKs on collagen I.
Several mutations have been described causing thrombocytopenia, including genes encoding transcription factors, signaling pathway proteins, proteins of unknown function in platelet production, and components of the cytoskeleton.30,31 Microthrombocytopenias in humans are rare and predominantly associated with mutations in genes encoding for direct or indirect interaction partners of the F-actin cytoskeleton (WAS,14 ARPC1B15 ). Recently, CARST (mutations in ADAP11,12 ) was described as a platelet disorder with a low platelet count and a decreased MPV. Although some CARST patients have a severe thrombocytopenia, others display moderately reduced or variable platelet counts over time.12,13 We show that constitutive ADAP-deficient mice, which are viable and do not show any sign of growth defects,22 also display a microthrombocytopenia with moderately reduced platelets counts (∼70% platelet count of control mice; Figure 1A). Interestingly, ADAP has been linked to actin cytoskeleton remodeling in macrophages,32 and we and others20 found that ADAP colocalizes with F-actin in platelets (Figure 1D). These findings corroborate the association of microthrombocytopenias and defects in actin-regulatory proteins, although it has to be mentioned that defects in actin-regulatory proteins resulting in disorganized DMS development can also lead to platelets of increased size.33 Even though a mathematical model was provided that platelet size is limited by cytoskeletal forces,34 the exact mechanisms leading to macro- and microplatelets in the vasculature are still only poorly understood.
Furthermore, CARST patients display variably increased bleeding tendency ranging from petechial rashes, mucosal and heavy menstrual bleedings to intra-abdominal bleeding from a ruptured corpus luteum,11 whereas mice exhibit increased rebleeding from wounds indicative of a role of ADAP in plug stability.19,20 In contrast to a previous report,21 we could not detect a splenomegaly in ADAP-deficient mice (supplemental Figure 5C), a finding that is in accordance with observations made in CARST patients.12,13 Furthermore, it was suggested that increased clearance of preactivated platelets might contribute to thrombocytopenia in CARST patients.11 We could not detect preactivated platelets in ADAP-deficient mice (Figure 2B), but instead found that their thrombocytopenia is caused by a combination of reduced platelet life span due to platelet clearance by macrophages (Figure 2A,F) and ectopic release of (pro)platelet-like particles into the BM compartment (Figures 3-5). Of note, ectopic release of (pro)platelet-like particles was still detectable in ADAP-deficient mice after macrophage depletion and platelet recovery on day 5 post clodronate administration (supplemental Figure 14). Interestingly, mice lacking Wiskott-Aldrich syndrome protein (WASp), Arp2/3, or Profilin 1 (Pfn1), all of which display a thrombocytopenia, also show ectopic release of (pro)platelet-like particles into the BM compartment,24,35,36 emphasizing the importance of F-actin organization in the terminal stages of platelet production. A multimolecular complex containing SLP-76, ADAP, Ena/VASP, Nck, and WASp has been identified in Raw264.7 macrophages, which mediates signaling between the FcRγ receptor and the actin cytoskeleton. SLP-76 might function as a central component in this complex, by binding both ADAP, which then recruits Ena/VASP and profilin, and Nck, which recruits WASp and Arp2/3.32 Similarly in activated T cells, ADAP associates with Ena/VASP family proteins and is present in complexes containing the proteins WASP, Nck, and SLP-76.37 Interestingly, in human platelets, adhesion to fibrinogen stimulated the association of SLP-76 with ADAP and VASP, thereby enabling SLP-76 to relay signals from the αIIbβ3 integrin to cytoskeletal reorganization.38 We speculate that this or a similar complex might also operate in MKs but this requires further investigation.
The reason for ectopic release of (pro)platelet-like particles into the BM compartment remains poorly understood. To exclude that other cell types contribute to the observed phenotype, we analyzed conditional Adapfl/f, PF4-cre mice restricting deficiency of ADAP to MKs and platelets. Adapfl/f, PF4-cre mice also displayed a microthrombocytopenia, reduced platelet life span (Adapfl/fl, t1/2: 40.6 ± 2.6 hours; Adapfl/fl, Pf4-Cre, t1/2: 31.2 ± 2.8 hours; P < .001), ectopic release of (pro)platelet-like fragments into the BM compartment, and recovery of the platelet count after macrophage depletion with clodronate (supplemental Figure 15), suggesting an MK-/platelet-specific phenotype. Recently, it has been demonstrated that Cdc42-dependent F-actin dynamics drive structuration of the DMS in MKs8 and lack of Cdc42 markedly impaired MK polarization in vitro.39 We found that ADAP-deficient MKs showed significantly impaired polarization in an in vitro–based assay (Figure 5C-D; supplemental Figure 11A) and we suggest that this defect might lead to nondirectional proplatelet formation as observed by immunostainings (Figures 3A, 4A) and electron microscopy (Figure 5B; supplemental Figure 10). Of note, ADAP deficiency did not affect the principal process of proplatelet formation in vitro (supplemental Figure 9A-B) and in vivo (Figure 4B; supplemental Videos 6-7).
Podosomes are actin-based structures involved in cell adhesion, migration, and extracellular matrix degradation. It has been proposed that altered podosome formation has an effect on effective transendothelial proplatelet formation and delivery of platelets into the bloodstream.27 In support of this, Wasp−/− and Pfn1−/− mice display thrombocytopenia and a strong defect in MK podosome formation.24,36 These data suggest a possible link between impaired podosome formation in vitro and platelet production defects characterized by ectopic release of platelets into the BM compartment in vivo. However, it has to be noted that alternative mechanisms may exist because phospholipase D–deficient MKs display abnormal actin rearrangement with almost abolished formation of podosomes, but normal platelet counts and no ectopic release of platelets.40 In this study, we found that ADAP-deficient MKs display severe defects in F-actin organization on collagen I and fibronectin matrices and significantly reduced podosome formation (Figure 6; supplemental Figure 13), whereas on collagen IV we could not observe defects in F-actin organization (supplemental Figure 13). Semeniak et al showed that fibronectin, collagen IV, and collagen I are present throughout the BM cavity.29 In addition, collagen I was found around larger arterioles and arteries, and subjacent to sinusoids, whereas collagen IV was localized to basement membranes of sinusoids. It was postulated that an inhibitory signal via GPVI with a branchpoint downstream of Src family kinases Fyn and Lyn attenuates proplatelet formation.29 Interestingly, in T cells 2 YDDV motifs of ADAP were shown to be phosphorylated by Fyn,16 suggesting a possible interaction of Fyn and ADAP also in MKs. Recently, it was demonstrated that β1 integrin activation on softer matrices is important for platelet production.41 Therefore, we hypothesized that altered actin reorganization in mutant MKs might lead to impaired integrin activation and consequently less adhesion to extracellular matrix proteins. Indeed, we found reduced activation of β1 integrins of Adap−/− MKs when spread on a collagen-coated surface (Figure 6F). This is in line with results from others42 and our own data (supplemental Figure 4) showing that ADAP deficiency affects integrin activation in platelets. However, it has to be mentioned that genetic ablation of the integrins β1 (Mx-cre mediated deletion) and α2, as well as kindlin and talin in the megakaryocytic lineage leads to no or only slightly reduced platelet counts in mice,43-47 suggesting that compensatory effects may exist for final release of platelets into the bloodstream. Thus, ADAP might have dual roles in proplatelet formation: (1) ADAP modulates F-actin organization as well as β1 integrin activation to establish proper adhesion of MKs to extracellular matrices and (2) might be involved in mediating an inhibitory signal to prevent proplatelet formation in MKs via interaction with Fyn. Further investigations are required to test the latter.
In conclusion, we found that ADAP deficiency affects actin polymerization and organization in platelets and MKs, and causes microthrombocytopenia in mice due to ectopic release of (pro)platelet-like particles into the BM compartment and decreased platelet life span. These results show similarities with previously described microthrombocytopenias associated with defects or lack in the actin-regulatory proteins WASp, Arp2/3, and Pfn1, pointing to an important axis of platelet disorders. We propose ADAP as a novel regulator of terminal platelet formation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sabine Schübert for excellent technical assistance, and the microscopy platform of the Bioimaging Center (Rudolf Virchow Center) for providing technical infrastructure. The authors also thank Irina Pleines for helpful comments and proofreading of the manuscript, Katrin G. Heinze for suggestions regarding imaging, Georgi Manukjan for assistance with flow cytometry analysis of the BM, and Harald Schulze for helpful comments and for providing the Rb polyclonal antibody to collagen I.
This work was supported by an Emmy Noether grant (BE5084/3-1 [M.B.]) and CRC/TR 240 (A01 [B.N.] and A06 [M.B.]) of the Deutsche Forschungsgemeinschaft (DFG).
Authorship
Contribution: M.S. performed experiments, analyzed data, and wrote the manuscript; J.M.M.v.E. and Y.S. performed experiments and analyzed data; P.N., B.N., D.S., and A.R. analyzed data and commented on the manuscript; M.B. supervised research, performed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Bender, Institute of Experimental Biomedicine Department I, University Hospital Würzburg, Josef-Schneider-Str 2, D15, 97080 Würzburg, Germany; e-mail: bender_m1@ukw.de.