TO THE EDITOR:
Myeloid malignancies are clonal proliferative diseases with shared but diverse phenotype characteristics; this classification includes (1) the myeloproliferative neoplasms (MPNs), polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF); (2) chronic myeloid leukemia (CML); (3) myelodysplastic syndrome (MDS); and (4) acute myeloid leukemia (AML).1 The etiologic basis of most myeloid malignancies is largely unknown. Although rare single-gene syndromes and predisposition disorders are associated with AML, MDS, and MPNs,2 they do not contribute significantly to the overall disease burden.3 Aside from these rare syndromes, there are limited data on the extent of familial aggregation of AML and MDS and their relationship to other MPNs. Understanding familial relative risks (FRRs) is clinically important because it allows for the discrimination of risk between individuals.2,4,5 In addition, these data are relevant to the design of research to identify susceptibility genes.6 The ability of previous studies to characterize the FRRs of myeloid malignancies has been limited, and no comprehensive analysis of the interrelationship between these diseases has been performed.7-10 To address these deficiencies, we have used the Swedish Family-Cancer Database to perform the largest population-based study of familial risks of myeloid malignancies to date, which included 93 199 first-degree relatives (FDRs) of 35 037 patients (supplemental Table 1, available on the Blood Web site).11
The Swedish Family-Cancer Database was created by linking information from the Multi-Generation Register, national censuses, the Swedish Cancer Registry, and death notifications.11 The Swedish Cancer Registry, established in 1958, is based on compulsory reporting of all cancers diagnosed in Sweden.12,13 We analyzed all primary cases of myeloid malignancies diagnosed between 1958 and 2015. Because MDS and MPNs not otherwise specified require codes from the International Classification of Diseases for Oncology, Second Edition, data for these malignancies could be ascertained only from 1993. Standardized incidence ratios (SIRs), as a measure of FRRs, were used to compare the cancer risks in FDRs of patients with a myeloid malignancy with the risk in the general population.14 All FDRs were observed from the date of birth, immigration, or the start of cancer-specific registrations in the database. Follow-up ended at diagnosis of cancer, date of death, emigration, or the end date of the registry. The SIRs (indirect standardization) were calculated as the ratio of observed cases to expected numbers of cases in the FDRs. To calculate the expected numbers of cases in the FDRs, age-, sex-, calendar year–, and disease-specific incidence rates in the population were multiplied by the corresponding person-years in FDRs. Estimates of 95% confidence intervals (CIs) were made assuming a Poisson distribution. Tests for trend in SIRs were performed by evaluating the likelihood function in collapsed person-time additive Poisson regression models with and without the inclusion of the variable. The lifetime cumulative risk was calculated based on the average life expectancy in Sweden in 2015 (82 years) and the following calculation: lifelong cumulative rate = sum of all age-specific incident rates; lifelong cumulative risk = 1–e–lifelong cumulative rate. To test for anticipation, the phenomenon in which a disease appears earlier in successive generations, we computed Kaplan-Meier estimates of risk by age and tested for homogeneity of parent and offspring strata using the log-rank test. This study was undertaken with the approval of the ethics committee at Lund University (Lund, Sweden) and conducted in accordance with the tenets of the Declaration of Helsinki.
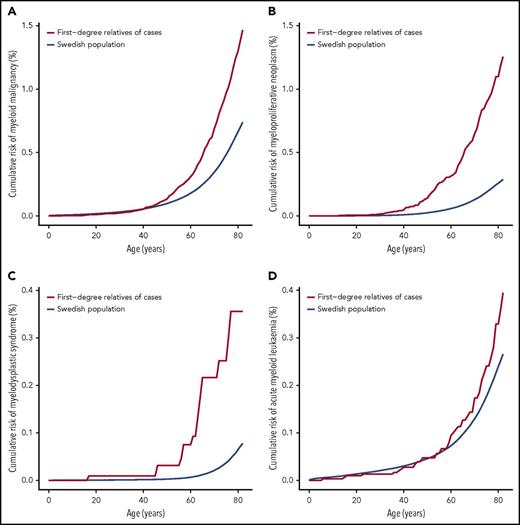
Overall, we observed an increased risk of all myeloid malignancies in FDRs of patients (1.99; 95% CI, 1.81-2.17). The association between family history and increased risk was statistically significant for AML (1.53; 95% CI, 1.12-2.04), essential thrombocythemia (6.30; 95% CI, 3.95-9.54), MDS (6.87; 95% CI, 4.07-10.86), and PV (7.66; 95% CI, 5.74-10.02). Between the myeloid malignancies, the strongest FRRs tended to occur for the same disease, although significant associations between diseases were noted (Table 1). We next examined FRRs for the same disease by age at diagnosis of the patient. A significantly increased FRR for younger cases when compared with older cases for all MPNs (6.46 vs 4.15), PV (10.90 vs 5.96), and MDS (11.95 vs 3.27) was observed (supplemental Table 2). The mean age of an MPN diagnosis was higher in parents of cases as compared with children of cases (70 vs 54 years; log-rank test P = .05). Although we cannot exclude the possibility of anticipation,15 this observation may reflect truncation bias.16 Sibling FRRs were significantly higher than parent-child risks for AML (3.29 vs 1.19; P = .0021) (supplemental Table 3). All 53 familial cases of PV were of a parent-child relationship (P = 1.0 × 10−5). We found little evidence that the sex of the patient or FDR had an effect on familial risk (supplemental Tables 4 and 5). We next examined FRRs by the number of affected relatives and found the FRRs of all myeloid malignancies and MPNs were significantly correlated with the number of affected FDRs. The SIRs for FDRs with 2 or more affected relatives for all myeloid malignancies and MPNs were 4.55 (95% CI, 2.08-8.64) and 17.82 (95% CI, 5.79-24.89), whereas the SIRs for FDRs with 1 affected relative were 1.96 (95% CI, 1.79-2.15) and 4.83 (95% CI, 4.14-5.60) (supplemental Table 6). Although the FRRs associated with these myeloid malignancies are among the highest known for cancers, these risks do not necessarily translate to a high absolute risk (Figure 1). However, markedly elevated cumulative risk estimates were obtained for all myeloid malignancies (4.4%) and MPNs (3.2%) in individuals with 2 or more affected FDRs (supplemental Table 6).
Cumulative risk for clonal myeloid diseases. (A) Myeloid malignancies overall, (B) myeloproliferative neoplasms, (C) myelodysplastic syndrome, and (D) acute myeloid leukemia.
Cumulative risk for clonal myeloid diseases. (A) Myeloid malignancies overall, (B) myeloproliferative neoplasms, (C) myelodysplastic syndrome, and (D) acute myeloid leukemia.
With more than twice the number of incident cases and 10 years longer follow-up than previous studies of the Swedish population,8,9 the increased study power has enabled us to demonstrate familial aggregation between different forms of myeloid malignancies. This population-based family cancer registry possesses robust familial relationship data with near complete case registration,12,13 allowing FRRs to be derived while avoiding biases introduced by case-control study designs. A previous analysis of the Swedish population used an additional registry to ascertain <15% MPN cases, which suggests there has previously been an underreporting of MPN cases.8 Potential underreporting has been ameliorated by our extended follow-up and improved statistical power. Although increased surveillance of relatives can bias familial risk estimates, such bias is not likely to occur in the general population over the long time period we have examined. Over recent decades, the diagnosis of hematologic malignancies has increasingly relied on molecular tests.1 Future work should therefore refine current risk estimates and identify familial risks associated with molecular subgroups.
Our findings indicate that inherited and environmental etiologic factors for myeloid malignancies are likely to be shared, and there is heterogeneity in the mechanisms by which such factors may exert their effects on different phenotypes. Consistent with early-onset tumors being more likely to have a genetic predisposition,6 for most of the myeloid malignancies, a relationship between familial risk and age at diagnosis was seen. The familial aggregation shown here justifies the continued application of gene-mapping approaches in high-risk families.17 Based on the paradigm of other cancers, including some MPNs,6,18,19 common genetic variation may also influence the development of the myeloid malignancies.
In summary, our findings provide evidence for genetic susceptibility to most myeloid malignancies as well as a shared genetic susceptibility between these malignancies. Furthermore, our data suggest that there are individuals, such as patients diagnosed at a young age and those with multiple affected FDRs, for whom counseling, gene testing, and surveillance may be appropriate. Finally, as recently advocated,2 such data may have implications for potential related stem cell donors.
Acknowledgments
This work was supported by grants from the German Cancer Aid, the Swedish Research Council (2014-2517, 2014-10134, and 2016-01176), and by Avtal om Läkarutbildning och Forskning funding from Region Skåne. A.S. is the recipient of a guest scientist fellowship from the German Cancer Research Center (DKFZ). R.S.H. is supported by funding from Bloodwise.
The online version of this article contains a data supplement.
Authorship
Contribution: A.S. and K.H. designed the study; K.H., K.S., and J.S. provided the data; A.S., S.C., and H.T. performed data extraction and statistical analysis; A.S., R.S.H., and K.H. drafted the manuscript; and all authors contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amit Sud, The Institute of Cancer Research, German Cancer Research Centre, 15 Cotswold Rd, London SM2 5NG, United Kingdom; e-mail: amit.sud@icr.ac.uk.