Abstract

Introduction
Aplastic anemia (AA) is a rare but potentially life-threatening disease that frequently occurs in older patients. Various therapeutic options can be proposed and data regarding the ideal first line treatment of AA in this ageing population remains scarce.
Methods
We conducted a retrospective nationwide multicenter study in France to examine current treatments for AA patients over 60 years old within a 10-year period (1/1/2007 to 12/31/2016). Our aims were to evaluate efficacy and tolerance of AA treatment, and to analyze predictive factors for response and survival. Patients who were diagnosed with AA by a bone marrow biopsy at the age of 60 or over were included in the study.
Results
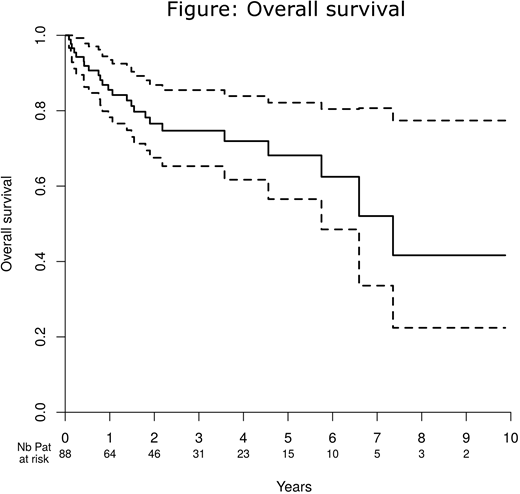
Over the course of a decade, 88 patients (median age 68.5) were identified in 19 centers, with a median follow-up of 32.1 months; 49% were women, the median Charlson comorbidity index was 2 (range 0-6), the median performance status was 1 (range 0-3), 21% had very severe (vSAA) and 36% severe AA. We analyzed 184 treatment lines which comprised ATG-CsA (33%, including 72% with horse ATG), CsA alone (14%), androgen alone (14%), eltrombopag alone (10%), CsA associated with androgen or eltrombopag (9%), and other treatments (20%). First-line treatment was ATG-CsA for half of patients. Comparisons of patients treated in first line with ATG-CsA, CsA or other treatments revealed that patients receiving ATG-CsA were significantly younger (66 years, vs. 71.5 vs. 71.5, respectively, p=0.007), more frequently female (61%, vs. 50% vs. 27%, p=0.02) and had a lower platelet count (8x109/L, vs. 12x109/L vs. 15x109/L, p=0.025). We found no difference with respect to weight, Charlson comorbidity index score, performance status or disease severity between first line treatment regimens. After first-line therapy, 32% of patients achieved a complete response (CR), and 15% a partial response (PR). After the 181 assessable treatment lines, 19% achieved CR and 19% a PR. Median time until best response was 151 days. The overall response rate (ORR) was 62% with ATG-CsA (70% as a first-line treatment), 35% with CsA alone (39% as first-line), 22% with eltrombopag, and 21% with androgen. The ORR in patients over 70 years receiving ATG-CsA (n=16) was 81% (50% achieving a CR). Responses were significantly better in first line and in patients with good performance status, as well as in those that had received ATG-CsA (ORR of 70% after first-line treatment). In a multivariable analysis using ATG-CsA as a baseline, we found that CsA alone (OR 0.35 (0.13;0.96), p=0.042), eltrombopag (OR 0.12 (0.03;0.54), p=0.0057) and androgens (OR 0.17 (0.05;0.58), p=0.0047) were all individually associated with lower response rates. The main complications were infections (grade III/IV, 35% of treatment lines including 9 deaths (5%)), and renal issues (grade-III/IV, 29%). ATG-CsA was associated with significantly more infectious complications (72% vs. 24%, p<0.0001), cardiovascular complications (32% vs. 15%, p=0.01) and acute kidney failure (43% vs. 22%, p=0.003) than other treatments. Patients aged 70 and over receiving ATG-CsA did not experience more complications than younger patients. Four clonal evolutions were recorded: two abnormal karyotypes (1 monosomy of chromosome 7 and 1 t(3;4)), one case of acute myeloid leukemia, and one case of myelodysplastic syndrome with 17% excess blasts. Three-year survival was 74.7% (median survival 7.36 years, Figure) and 24 patients died (nine infections, five deaths in palliative care or after active treatment had finished, four hemorrhagic complications, and six miscellaneous causes). Age (OR 1.07 (1.01;1.14), p=0.03), Charlson comorbidity index (HR 1.34 (1.07;1.67), p=0.01) and vSAA (HR 3.67 (1.51;8.91), p=0.004) were independently associated with mortality.
Conclusion
Our study showed a significantly better ORR with ATG-CsA than other regimens for treatment of AA in elderly, with more complications but no more death. Age per se is not a limiting factor for treatment with ATG-CsA: this regimen should be used as first-line treatment in elderly patients if they have a good performance status and low comorbidity index score. Among patients with adverse performance status or comorbidities contra-indicating the use of ATG, CsA alone or in combination may be safely used. Other strategies might be reserved for later courses of treatments. Supportive care may have a great impact on survival in this population.
Ades:JAZZ: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; silent pharma: Consultancy; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Peffault De Latour:Pfizer Inc.: Consultancy, Honoraria, Research Funding; Amgen Inc.: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract