Abstract
Background and Aim: NK cell effects are mediated by Killer Immunoglobulin-like Receptor (KIR) inhibition. There are few reports on the protective role of KIRs against relapse in myeloma(MM) (Gabriel et al Blood 2010, Kröger et al leukemia 2011). However, which NK cells or for whom will be more effective and the role of individual differences in this mechanism is indefinite. In this study we aimed to accomplish cytotoxicity against both MM cells and human myeloma cell lines (HMCL) and to compare the cytotoxicity achieved with CB versus autologous NK cells as well as to investigate the influence of NK cell KIR geno/phenotypes.
Patients and Methods: A total of 18 patients with refractory/relapsed MM were included in this study.
Cell culturing: CD138+ PC isolated from fresh bone marrow aspirates of relapsed MM patients or HMCL (U266, RPMI8226 and H929) using RosetteSep™ Human Multiple Myeloma Cell Enrichment Cocktail (StemCell Technologies) were subjected to cytotoxicity assays with CB derived or autologous PB derived NK cells expanded in the presence of IL-15 (10ng/ml) for 2-8 days.
Phenotyping: Effector cells (autologous or CB-NK) were analyzed by flow cytometry using CD45, CD3, CD16, CD56, CD158a (KIR2DL1), CD158i (KIR2DS4) and CD158b1/b2 (KIR 2DL2/2DL3) monoclonal antibodies.
Cytotoxicity assays:3,3 dioctadecyloxacarbocyanine perchlorate (DiO) dye was used to distinguish target cells (PC) from effector cells (NK). PC and NK cells were mixed at an 1:10 target: effector cell ratio and inoculated with Propidium Iodide (PI) in order to counter stain the dead cells. Naive PC were used as controls. Following 120 mins of incubation, PC death was evaluated using flow cytometry. After incubation of target cells (PC) with effector cells (NK cells), cytotoxic effects were determined by subtraction of NK mediated PC death from spontaneous PC death.
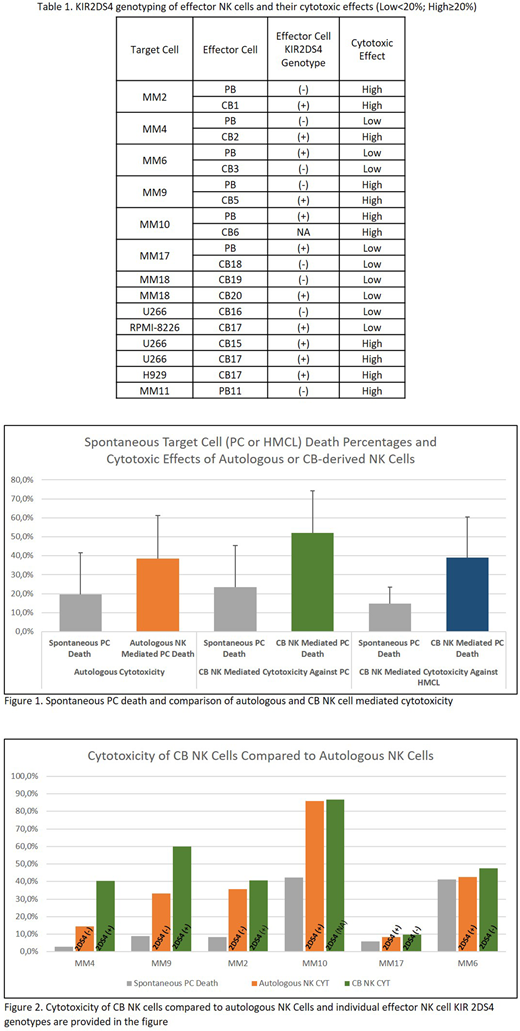
Results: Following IL-15 expansion NK cells were found to express the CD16+56+3- phenotype. Eighteen MM patient PCs and six different HMCL samples were used in cytotoxicity assays(Table 1). In vitro PC viability was less than 45% in four samples and could not be analyzed in the study. Following incubation of PC wo NK cells, spontaneous PC death ratio was found to be 22.6% (min-max: 3%-42.3%). Twelve cytotoxicity assays targeting six MM PC were performed with autologous and CB-derived NK cells. Autologous NK and CB NK mediated cytotoxicity median rates were found to be 19.0% (range: 1.4% - 43.6%) and 31.3% (range: 2.4% - 51.2%), respectively. Additionally, CB-NK cells were evaluated against HMCL samples (four U266, one RPMI8226 and one H929). Median cytotoxicity against HMCL was 27.4% (range: 5.6% - 52.1%). Highest cytotoxicity against MM PC (MM-9) and HMCL (H929) were achieved by CB NK cells (51.2% and 52.1 % respectively). There was no cytotoxic effect against PCs of patients MM6 and MM10 (Figure 1-2).
Flow-cytometric phenotyping of effector NK cells showed consistency (10/13) with genotypes. Since only one (CB19) with 19% CD158i positivity was PCR negative, a %20 cut-off rate was chosen. Both CD158a (KIR2DL1) and CD158b1/b2 (KIR 2DL2/2DL3) were positive among all patients and no significant correlation with cytotoxic effects was detectable. However out of five high cytotoxic reactions three NK cells were 2DS4 (+). While out of eight assays which resulted with low cytotoxicity only three NK cells were positive for the activating KIR 2DS4. When we analyzed CB NK cells against HMCL U266, three CB NK cells two of which were 2DS4(+) showed high cytotoxicity, while the 2DS4(-) CB NKs did not. The only assay targeting RPMI-8226 which is known to be a NK resistant HMCL, KIR2DS4(+) (CB17) derived NK cells were not sufficiently cytotoxic.
Conclusion: Allogeneic CB NK cells were able to induce higher cell kill than autologous PB-derived NK cells. KIR types of autologous NK cells were not predictive for cytotoxic efficacy. Lacking KIR2DS4 in CB NK cells was correlated with less cytotoxicity. Additionally, KIR2DS4(+) CB NK cells were found to be highly cytotoxic against both PC and HMCL. NK cells may be an effective alternative due to its profound clinical advantages such as matching for HLA or KIR ligand is not required. Furthermore, if confirmed by others, certain KIR haplotypes (ie Haplotype A and KIR2DS4 +) may offer better therapeutic potential.
Acknowledgment: The study has been supported by Scientific and Research Council of Turkey (TUBITAK grant no: 115S579) and Turkish Academy of Sciences.
Beksac:Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen,Janssen-Cilag,Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.