Abstract
Conditioning chemotherapy is used to deplete hematopoietic stem cells in the recipient's marrow prior to a bone marrow transplant to facilitate engraftment of donor cells. While effective, some major issues with chemotherapy remain which includes off-target genotoxic effects increasing the risk of secondary malignancies. These complications are compounded in disease settings that arise from a deficit in DNA repair pathways where cytotoxic treatments can result in oncogenic transformation, such as Fanconi anemia (FA). FA is an inherited bone marrow failure disorder resulting from an intrinsic defect in DNA repair affecting approximately 130,000 children each year. Currently mutations in 22 genes have been implicated in the pathogenesis of FA. While novel gene-therapy based protocols are showing early promise for this patient populations, the standard treatment for the hematologic complications for FA is a bone marrow transplant. However, secondarily to an underlying sensitivity to DNA damage, these patients are unable to receive standard myeloablative conditioning, which can reduce the efficiency of reconstitution. Avoiding alkylating agents could improve outcomes and success rates in these patients. Furthermore, this methodology could be translated to the allogeneic bone marrow transplantation setting, decreasing the toxicity of this treatment modality.
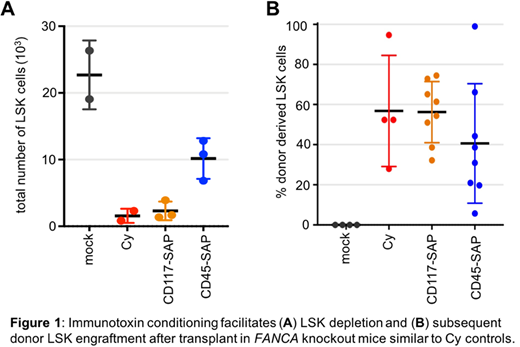
Our approach for characterizing an alternative conditioning regimen for FA patients utilizes immunotoxin conjugates specifically targeting hematopoietic stem cell populations. These non-genotoxic antibody-based drug conjugates utilize saporin (SAP), a ribosomal toxin, to eliminate cells in a targeted manner while leaving the remainder of the marrow compartment intact. Antibodies targeting either CD45 or CD117 were used in a mouse model of FA where expression of their FANCA gene, one of the most common mutations in humans, has been knocked out. Mice conditioned with either of the drugs received various doses of whole marrow from healthy heterozygous littermates. On the day of transplant, mice conditioned with either immunotoxin demonstrated significantly reduced LSK stem cell populations in the marrow similar to cyclophosphamide (Cy) controls (Figure 1A).
Peripheral engraftment of donor cells was monitored for 6 months, after which mice were sacrificed for complete analysis of engraftment in the bone marrow compartment. No significant difference was observed in engraftment between Cy and immunotoxin conditioned mice (Figure 1B), and all treatment groups exhibited robust multilineage reconstitution in a cell dose dependent manner. Additionally, Cy treated mice demonstrated greater and sustained weight loss and lower gastrointestinal losses compared to immunotoxin treated mice. Ongoing clonal analysis studies of engrafted cells has demonstrated polyclonal reconstitution, indicating a large number of donor stem cells are actively contributing to hematopoiesis.
In this study, we demonstrate that non-genotoxic conditioning approaches both facilitate multilineage engraftment of donor marrow and significantly deplete host hematopoietic stem cell populations. These are crucial since persistence of host hematopoiesis could eventually result in clonal evolution and leukemogenesis in post-transplant FA patients. Achieving both of these conditions through targeted elimination with immunotoxin conjugates represents a major advancement in bone marrow transplantation for FA. We now are initiating studies using immunotoxin-based conditioning for the transplantation of gene-modified syngeneic stem cells and allogeneic cells. We think these studies will inform future clinical trials and provide the groundwork for the next-generation of therapy for FA patients.
Hartigan:Magenta Therapeutics: Employment. Palchaudhuri:Harvard University: Patents & Royalties; Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Boitano:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Cooke:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Kiem:Homology Medicine: Consultancy; Magenta: Consultancy; Rocket Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.