Abstract
Background: Veno-Occlusive Disease (VOD) or Sinusoidal Obstruction syndrome (SOS), is a rare but potentially life-threatening complication after Hematopoietic Stem Cells Transplantation (HSCT). The clinical diagnosis of VOD relies on signs and symptoms, such as weight gain, painful hepatomegaly and jaundice, directly correlated to the development of sinusoidal portal hypertension (PH). It has been demonstrated that the earlier is the treatment start better the outcome. For this reason, non-invasive diagnostic tools of VOD/SOS diagnosis are needed. Ultrasound (US) Elastography techniques, assessing the Liver Stiffness Measurements (LSM), have been demonstrated to be able to assess PH degree. The role of LSM in VOD/SOS has not yet been reported in the setting of adult HSCT.
Methods: We report the results of a prospective single center study evaluating the role of LSM assessed by Transient Elastography (TE), in adult patients undergoing allogeneic HSCT for haematological malignancies since April 2016 at the Institute of Hematology "L. and A. Seràgnoli", Sant'Orsola-Malpighi University Hospital, Bologna, Italy. The exclusion criteria were patients with Body Mass Index (BMI) over 40 kg/m2, ascites and diagnosis of advanced chronic liver disease (ACLD) at pre-HSCT assessment. LSM were assessed bedside of patients by TE, using the FibroScan® apparatus with "M" probe (Echosens, Paris, France), after overnight fasting and after a complete abdominal US exam, before the start of conditioning regimen and then at 3 time points after transplant (on days +9/10, +15/17 and +22/24).
Results: Over the study period, among of 89 patients referred to our centre 78 patients fulfilled the inclusion criteria. Median age was 54 years (40-60). The most frequent indication was Acute Myeloid Leukemia (44.9%). 66.7% of patients received a myeloablative conditioning while 33.3% received a RIC regimen. 15.4% were transplanted from HLA identical sibling donor, 74.4% from unrelated donor, 3.8% from Haplo and 6.4% from Cord Blood. The baseline median LSM was 4.2 kPa (3.8-5.3).
During the study, 4 out 78 patients (5.1%) met clinical diagnostic criteria of VOD/SOS. The median day of VOD/SOS diagnosis was +17 (+1 - +29). Most of the VOD/SOS were Severe/Very severe (3/4), one of them leading to Multiple Organ Failure and death. Accordingly with EBMT criteria, the total number of pre-HSCT VOD/SOS risk factor was significantly higher in patients who developed VOD/SOS. We also valuated the US signs, according to Lassau criteria, in patients with VOD/SOS: 3 out 4 patients had ≥3 Grey-scale US morphologic Criteria and none presented ≥3 US Colour Doppler Criteria. Only portal vein diameter >12 mm was presented in all patients with VOD/SOS diagnosis.
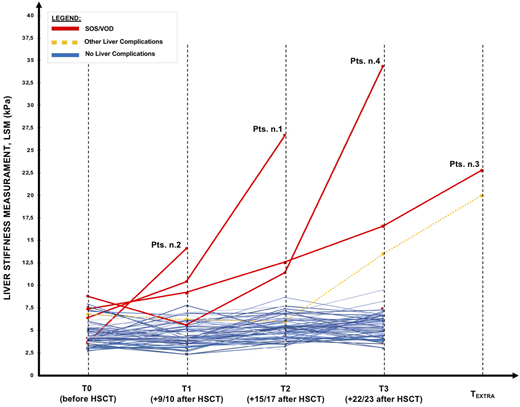
LSM values for each time points were reported in Figure 1; LSM by TE showed increased values in all patients who developed VOD/SOS (red continuous lines). Pre-HSCT (T0) LSM tended to be slight (p-value 0.079) higher (6.9 kPa vs 4.2 kPa) in patients who developed VOD/SOS. In these patients, the LS values increased before VOD/SOS clinical diagnosis, with values significantly different from pre-HSCT and from the previous assessments, anticipating from 2 to 12 days the clinical VOD/SOS development. After starting VOD/SOS specific treatment (defibrotide and diuretics) LSM values consensually decreased to pre-transplant values within 2-4 weeks.
In univariate analysis an LSM increase after HSCT was significantly associated with VOD/SOS development (OR: 1.837; 95%CI [1.1107- 3.0384], p<0.01); indeed, ROC analysis of LSM increase over pre-HSCT assessment resulted an AUC of 0.997 with sensitivity of 75% and specificity of 98.7%. For instance, an increase ≥+10 kPa over pre-HSCT assessment showed a sensitivity of 100% and specificity of 98.7% for VOD/SOS diagnosis.During the study, 24 out 74 patients developed other HSCT-related liver complications: for them the increase of LSM after HSCT was not significantly different (p: 0.677) compared to patients who did not developed.
Interpretation: LSM by TE showed significantly increased values in all patients who developed VOD/SOS, anticipating by some days the clinical criteria for VOD/SOS diagnosis. By our knowledge this is the first prospective study focused on the use of LSM for early prediction of VOD/SOS. Further validation of these data are warranted to confirm the diagnostic role and the predictive value of LSM in the VOD/SOS development.
Zinzani:Takeda: Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Speakers Bureau; MSD: Honoraria, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cavo:GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.