Abstract
BACKGROUND
The use of haploidentical stem cell transplantation (haploSCT) in patients without suitable matched sibling/unrelated donors has greatly improved access to potentially curative treatment for myeloid malignancies [Acute Myeloid Leukemia (AML), Myelodysplastic Syndrome (MDS), Myelofibrosis (MF)]. We aim to investigate the efficacy of haploidentical unmanipulated peripheral blood stem cell (PBSC) transplantation in 44 patients with myeloid malignancies using a reduced toxicity conditioning regimen with post-transplant cyclophosphamide (PTCy), anti-thymocyte globulin (ATG), and cyclosporine (CsA) to prevent rejection and graft versus host disease (GVHD).
METHODS
Forty-four adults with myeloid malignancies (AML=31, MDS=8, MF=5) underwent haplo-transplants between August 2016 and February 2018 at our centre. Conditioning included fludarabine (30mg/m2/day on day -5 to -2), busulfan (3.2mg/m2/day on day -3 and -2), and total body irradiation (200 cGy) on day -1. Unmanipulated PBSCs were infused on day 0. GVHD prophylaxis included ATG (4.5 mg/kg over day -3 to -1), PTCy (50mg/kg/day on day +3,+4), and CsA from day+5.
Baseline clinical information was collected through retrospective chart review. Last follow up was June 2018. Median follow up for patients known to be alive was 5 months (range 0-15). The main variables of interest were overall survival (OS), progression-free survival (PFS) and non-relapse mortality (NRM) at 6 months and 1 year. The cumulative incidences (CI) of acute and chronic GVHD were calculated accounting for death and relapse as competing risks.
RESULTS
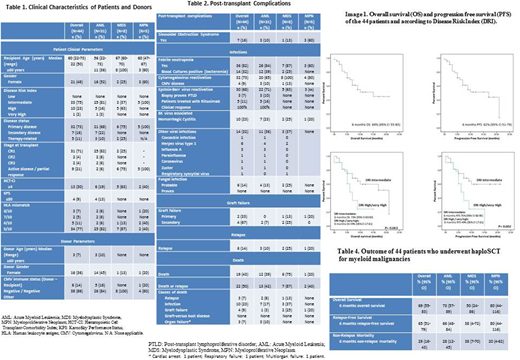
Baseline characteristics are shown in Table 1. Median time to neutrophil and platelet engraftment was 17 days (range 10-43) and 23 days (range 7-98) respectively. Post-transplant complications are described in Table 2. Two (5%) patients had primary graft failure and four (9%) had secondary graft failure. The cumulative incidence (CI) of grade II-IV and III-IV acute GVHD at day +100 was 35.7% (95% CI 23-48) and 5.7% (95% CI 1.5-14) respectively. The CI of moderate chronic GVHD at 6 months was 5.9% (95% CI 1.5-14.8) and the CI for severe chronic GVHD was 0%. Thirty-two (73%) patients had CMV reactivation, four (9%) patients had CMV disease. EBV reactivation was documented in 30 (68%) recipients. Five (11%) required Rituximab treatment for EBV titres >1x10E6 or biopsy proven Post-Transplantation Lymphoproliferative Disorder (PTLD) with 100% response. Ten (23%) patients had BK virus related hemorrhagic cystitis and 14 (42%) patients were diagnosed with other viral infections during follow up. Finally, 6 (14%) patients had probable lung fungal infection. Six (14%) relapses occurred during follow up. Main cause of death was infection in 10 (23%) patients, followed by graft failure in 4 (9%) cases, relapse in 3 (7%) and organ failure in 3 (7%). Six months OS, PFS and NRM are exposed in Table 2 and Figure 1. Significant differences in OS were found according to Disease Risk Index (DRI) (p=0.02) (Figure 1).
CONCLUSION
HaploSCT with unmanipulated PBSCs provides a route to potentially curative therapy for patients without access to conventional donor sources. In vivo T-cell depletion leads to low incidence of acute and chronic GvHD but high rates of infectious complications, particularly viral infections.
Lipton:Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.