Abstract
Cyclophosphamide (CY) toleration has been associated with low rates of graft versus host disease (GVHD) and non-relapse mortality after both HI and MR HSCT. At Jefferson, both recipient types undergo a 2 step approach to HSCT in which after myeloablative (MYA) or reduced intensity (RIC) conditioning, patients receive a fixed dose of 2 x 108/kg donor CD3+ T cells (DLI), followed 2 days later by CY daily x 2 for bidirectional tolerization. A CD34-selected stem cell product containing < 5 x 104/kg untolerized CD3 cells is infused 24 hours after CY. Mycophenolate mofetil and tacrolimus are started on day-1 for GVHD prophylaxis. The consistency of cell doses, conditioning, and GVHD prophylaxis in the approach provides an optimal platform for comparing patient outcomes. Because tolerance induction and sustained engraftment in CY-based HSCT approaches depends on elimination of donor and host alloreactive T cells, we hypothesized that engraftment in this model would be different between HI and MR recipients based on the lower proliferation of alloreactive T cells in major histocompatibility (MHC) MR versus HI donor-recipient pairs.
Data for 292 consecutive patients (232 HI, 60 MR) treated on a 2-step trial were evaluated for incorporation into this analysis. To be included, patients were required to be in remission for 3 months post HSCT and have available d+28 and d+90 peripheral blood T cell chimerism data. Seven HI patients with graft rejection, 5/232 with early (2%-all MYA) and 2/232 with late (0.1%-1 MYA and 1 RIC), were not included in the analysis. No MR patients developed graft rejection. Days to ANC > 500 cells x 103/ul, CD3/4 and CD3/8 counts at d+28 and d+90, peak fever after DLI infusion as a rough measure of alloreactivity, and number of patients requiring post HSCT donor lymphocytes for mixed chimerism were also examined. Independent samples T test was used for chimerism and peak fever comparisons.
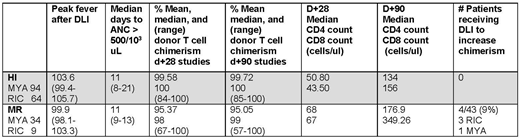
The final study group contained 201 patients treated on a 2 step MYA [12 Gy total body irradiation (TBI)] or RIC (fludarabine/TBI based) regimen. Patients had various hematological malignancies and 6 had aplastic anemia. Data is contained in the table.
Mean T cell chimerism was higher in HI versus MR recipients at d+28 and d+90 (p= 0.001 both time points). In 3 MR recipients, donor T cell chimerism was < 70% through d+90. Four (9%) MR versus no HI recipients received post HSCT DLI for mixed chimerism in the absence of recurrent disease. Chimerism differences did not affect time to myeloid engraftment or lymphoid reconstitution. While higher median peak temperatures after DLI were observed in HI versus MR recipients, some patients in the latter population were also highly febrile. Those MR recipients with higher fevers had a higher mean donor engraftment percentage at d+28 than those with lower fevers, but the difference was not significant (96.61% vs 93.95%, p=0.193).
The data presented here supports the concept that MHC mismatching in HI HSCT is associated with a higher frequency of alloreactive T cells in the DLI which more efficiently eliminate residual host lymphocytes prior to CY administration resulting in more complete donor lymphoid chimerism. Interestingly, further support for this notion was provided by the MR recipient data where higher fevers, a surrogate for more robust immunologic activation, translated into the more rapid achievement of full donor chimerism. This finding was potentially due to higher alloreactivity between some MR recipient/donor pairs from non-MHC polymorphisms. Persistence of small numbers of host lymphocytes, which have also been rendered tolerant by CY administration, does not, in and of itself, lead to graft failure/rejection. However, concerns that low degree of chimerism might contribute to higher risk of relapse led to administration of post-transplant DLIs to push donor lymphoid chimerism closer to 100%. The impact if any, of delayed complete chimerism on GVHD and relapse requires further evaluation and may dictate changes to the 2 step regimen for the MR group.
Porcu:Innate Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.