Abstract
Introduction : High total metabolic tumor volume (TMTV) measured on 18F-FDG PET/CT before R-CHOP has been shown to be significantly associated with worse progression-free survival (PFS) and overall survival (OS) in patients with diffuse large B-cell lymphoma (DLBCL; Cottereau et al. Clin Cancer Res. 2016;22:3801-9) . The REMARC study (NCT01122472) is an international, multicenter, double-blind, randomized phase III trial that assessed lenalidomide (LEN) maintenance therapy versus placebo (PBO) in 650 patients responding to R-CHOP. With a median follow-up of ~40 months, independent review demonstrated that 2 years of LEN maintenance therapy significantly improved progression-free survival (PFS); median was not reached in the LEN arm vs 58.9 months in the PBO arm (HR=0.71 [95% CI, 0.54-0.93]; p=0.0135; Thieblemont et al. J Clin Oncol. 2017;35:2473-81).
Methods: For these analyses, patients enrolled in the REMARC trial who had baseline PET/CT before R-CHOP (not mandatory per study protocol) with available fused images and end of treatment PET/CT were included. Total metabolic tumor volume (TMTV, defined as the sum of the regions of the local tumors with FDG uptake) was measured on baseline PET/CT with the 41% SUVmax thresholding method using the free semiautomatic software Beth Israel Fiji20 (http://petctviewer.org). The optimal TMTV cut-off to PFS (per FDA censoring rule) and overall survival (OS) was determined by Receiver Operating Curve (ROC) curves and X-tile analyses. Survival was estimated using Kaplan Meier (KM) curves. Multivariable analysis were performed with descending Cox model including TMTV, IPIaa, treatment arm and PET/CT response evaluated by Deauville criteria. Analyses were performed on the evaluable population and separate arms
Results: 228 of 650 REMARC patients had TMTV data available for analysis, including n=108 in the PBO arm and n=120 in the LEN arm. Clinical characteristics were similar to the overall population. The median baseline TMTV was 295 cm3 (Q1-Q3, 99-702). After a median follow-up of 51.6 mo, 4y-PFS was 73% and 4y-OS was 85%. The optimal TMTV cut-off determined by ROC was 300 cm3 for PFS and OS.
Patients with TMTV >300 vs ≤300 cm3 presented with worse ECOG performance status (ECOG ≥2: 19% vs 9%, p=0.034), higher Ann Arbor stage (stage III-IV: 95% vs 86%, p=0.042), more extra-nodal sites (>1: 65% vs 38%, p<0.001), more frequently elevated LDH (76% vs 43%, p<0.001), higher IPI (IPI 3-5: 87% vs 51%, p<0.001), and higher aaIPI (aaIPI 2-3: 76% vs 34%, p<0.001).
In all evaluated patients, a significant impact of TMTV for cut-offs of >300 vs ≤300 cm3 was observed for PFS (HR=2.09; 95% CI, 1.22-3.69) and OS (HR=2.99; 95% CI, 1.44-6.18). Patients with high TMTV >300 cm3 vs low TMTV ≤300 cm3, respectively, had a 4-year PFS of 57% vs 73% and OS of 70% vs 88%. These results were more disparate when a higher TMTV cut-off of >1000 was applied. In multivariate analysis, only TMTV maintained an independent prognostic value.
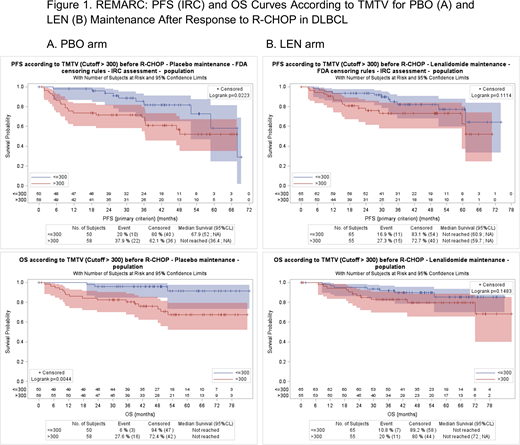
The prognostic impact of TMTV >300 vs ≤300 cm3 on PFS (HR=2.4; 95% CI, 1.1-5.22) and OS (HR=5.0; 95% CI, 1.4-17.) was maintained in the PBO arm (Figure 1A). In contrast, when the analysis was focused on patients in LEN arm, TMTV >300 vs ≤300 cm3 lost its prognostic impact on PFS and OS. In the LEN arm, 4-year PFS and OS did not differ significantly between patients with high and low TMTV (Figure 1B).
Conclusion: TMTV measured on baseline PET/CT is a strong prognosticator of outcome in DLBCL, even in patients in response after R-CHOP. High TMTV at baseline was significantly associated with worse PFS and OS in patients receiving PBO following a response to R-CHOP in the REMARC study. Interestingly, LEN maintenance reduces the negative impact of high baseline TMTV on survival in patients with DLBCL
Casasnovas:takeda: Consultancy; merck: Consultancy; MSD: Consultancy; Roche: Consultancy; Gilead Sciences: Research Funding; Roche: Honoraria; Takeda: Honoraria; Merck: Honoraria; Gilead Sciences: Honoraria; Janssen: Honoraria; Celgene: Honoraria; MSD: Honoraria; Roche: Research Funding; Gilead Sciences: Consultancy; Janssen: Consultancy. Tilly:Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Membership on an entity's Board of Directors or advisory committees. Feugier:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ribrag:Infinity: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Amgen: Research Funding; Roche: Honoraria, Other: travel; MSD: Honoraria; BMS: Consultancy, Honoraria, Other: travel; epizyme: Consultancy, Honoraria; NanoString Technologies: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; argenX: Research Funding; pharmamar: Other: travel; Incyte Corporation: Consultancy. Macro:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress. Morschhauser:Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy; Janssen: Other: Scientific Lectures; Roche: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Trotman:Janssen: Other: Unremunerated member of Ad Board, Research Funding; F. Hoffman-La Roche: Other: Travel to meeting, Unremunerated member of Ad Board, Research Funding; Takeda: Other: Unremunerated member of Ad Board; Celgene: Other: Unremunerated member of Ad Board, Research Funding; PCYC: Research Funding; Beigene: Research Funding. Godmer:CELGENE: Other: Invitation to congress. Salles:Servier: Honoraria; Novartis: Consultancy, Honoraria; Morphosys: Honoraria; Servier: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board, Research Funding; Acerta: Honoraria; Merck: Honoraria; Janssen: Honoraria, Other: Advisory Board; Pfizer: Honoraria; Epizyme: Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Gilead: Honoraria, Other: Advisory Board; BMS: Honoraria, Other: Advisory Board; Takeda: Honoraria; Amgen: Honoraria; Abbvie: Honoraria. Coiffier:CELGENE: Consultancy, Membership on an entity's Board of Directors or advisory committees; MUNDIPHARMA: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees; MORPHOSYS: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees. Meignan:F. Hoffman-La Roche Ltd: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.