TO THE EDITOR:
Chimeric antigen receptor (CAR) T-cell therapy has significantly improved the outcome of patients with relapsed/refractory large B-cell lymphoma, resulting in durable remissions in ∼40% of heavily pretreated patients.1-4 Of interest, patients with concomitant active hepatitis B virus (HBV) or hepatitis C virus (HCV) infection are generally excluded from clinical trials of CAR T-cell therapy in aggressive B-cell lymphoma, out of concern for viral reactivation and fulminant hepatitis. As a consequence, the safety of CAR T-cell therapy in patients with B-cell lymphoma and concomitant HBV or HCV infection, a quite common scenario, remains completely unexplored.5,6
Three patients with relapsed/refractory diffuse large B-cell lymphoma and concomitant HBV or HCV infection have been treated with axicabtagene ciloleucel (axi-cel), an anti-CD19 CAR T-cell therapy, at our institution between 2016 and 2018. This retrospective study was approved by the institutional review board of the University of Texas MD Anderson Cancer Center and conducted in accordance with the principles of the Declaration of Helsinki.
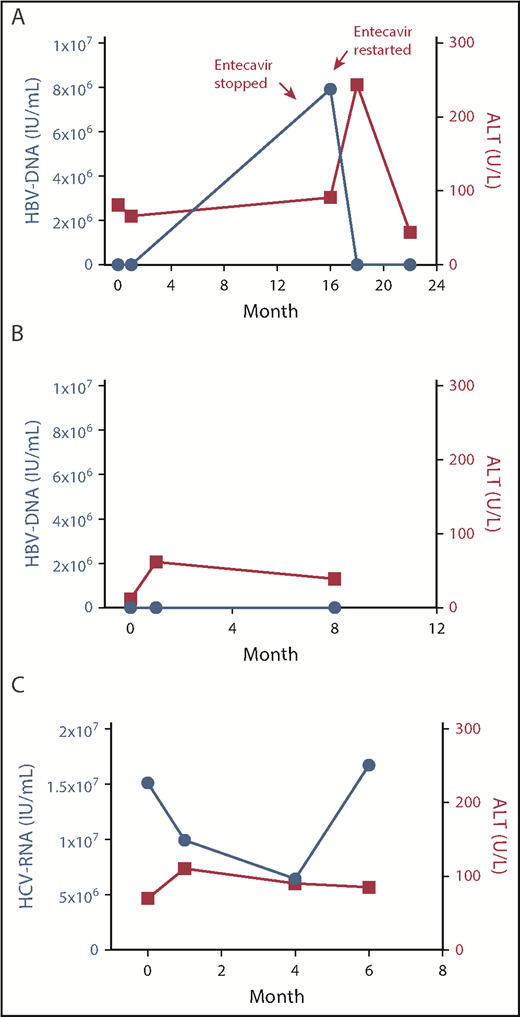
The first patient was a 40-year-old black woman who relapsed after 4 lines of therapy and autologous stem cell transplant. At the time of evaluation for axi-cel, the patient was found to be negative for hepatitis B surface antigen and positive for hepatitis B core antibody, with detectable HBV DNA by polymerase chain reaction (<20 IU/mL) but negative HBV nucleic acid testing (Table 1). Prophylaxis with entecavir 1 mg by mouth (postoperatively) daily was started, and axi-cel was given 1 week later. Treatment was complicated by grade 2 cytokine release syndrome (CRS)7 and grade 4 CAR-related encephalopathy syndrome (CRES), characterized by benzodiazepine-refractory tonic-clonic seizure requiring intubation. The patient required triple antiepileptic therapy, including levetiracetam, phenytoin, and lacosamide, with resolution of encephalopathy and self-extubation after 7 days. Antiepileptics were slowly tapered and completely discontinued over the course of 7 months, with no seizure recurrence. The patient achieved complete response (CR) by 1 month, ongoing after 31 months of follow-up. No significant viral reactivation or increase in alanine aminotransferase (ALT) levels was observed during CRS or CRES (Figure 1A). Bilirubin total peaked to 2.2 mg/dL (direct 1.5 mg/dL) 2 days after CAR T-cell therapy infusion, and normalized in 6 days. However, the patient self-discontinued antiviral prophylaxis 13 months after axi-cel infusion, and had HBV DNA reactivation (79 million IU/mL) 3 months afterward, which was successfully treated with re-initiation of entecavir.
Trends in HBV DNA/HCV RNA and ALT after CAR–T-cell infusion. HBV/HCV DNA and ALT levels are shown. The x-axis represents time from axi-cel infusion (months). Actual DNA/ALT measurements are represented by dots. (A) Patient 1. (B) Patient 2. (C) Patient 3.
Trends in HBV DNA/HCV RNA and ALT after CAR–T-cell infusion. HBV/HCV DNA and ALT levels are shown. The x-axis represents time from axi-cel infusion (months). Actual DNA/ALT measurements are represented by dots. (A) Patient 1. (B) Patient 2. (C) Patient 3.
The second patient was a 54-year-old Asian man who relapsed after 2 lines of therapy (Table 1). The patient had a known history of chronic hepatitis B and was on antiviral prophylaxis with tenofovir 25 mg postoperatively daily, which was started with initiation of chemoimmunotherapy 12 months prior. At the time of evaluation for axi-cel therapy, HBV DNA was detectable at low titer (<10 IU/mL), and tenofovir was continued at the same dose. Axi-cel therapy was complicated by grade 3 CRS and grade 2 CRES. No significant viral reactivation or ALT/bilirubin elevation was observed (Figure 1B). The patient achieved CR that is ongoing after 8 months of follow-up and he remains on tenofovir.
The third patient was a 60-year-old white man who relapsed after 2 lines of therapy (Table 1). The patient had a long-lasting history of chronic hepatitis C that did not respond to interferon and ribavirin therapy 25 years before presentation. At the time of evaluation for axi-cel therapy, the patient’s HCV RNA was 15.1 million IU/mL and ALT was 70 U/L. Axi-cel infusion was complicated by reversible grade 3 CRS and grade 3 CRES. No significant increase in HCV RNA or ALT/bilirubin levels were observed (Figure 1C). The patient achieved a CR 1 month after the infusion, which is ongoing at 6 months. Despite recommendation, the patient declined alternative anti-HCV therapeutic options.
This is the first report of the safety of CAR T-cell infusion in patients with B-cell lymphoma and concomitant HBV or HCV infection. No fulminant hepatitis was observed in any of the 3 patients receiving axi-cel. Of interest, no patient in this study had concomitant liver cirrhosis, and no data are available to support the safety of the use of CAR T-cell therapy in patients with this condition, particularly in case of concomitant liver insufficiency and/or portal hypertension.
Both entecavir and tenofovir were shown to be effective antiviral prophylaxis in the 2 patients with chronic hepatitis B. These 2 agents have shown to be less likely to cause drug resistance and more likely to result in viral suppression compared with lamivudine.8 Retrospective studies have shown that tenofovir may be more effective than entecavir in patients with positive hepatitis B e-antigen, but this was not tested in the 2 patients included in this series.9 As B-cell aplasia can be prolonged and there are no data at this time on T-cell immune reconstitution after anti-CD19 CAR T-cell therapy, antiviral prophylaxis may need to be continued long-term, as suggested by HBV reactivation experienced by the patient who self-discontinued entecavir 1 year after therapy.
The small sample size does not allow us to determine any association between concomitant HBV or HCV infection and CRS or CRES. Although the etiology of these entities remains to be fully clarified, both seem to be cytokine-driven, with interleukin 6 (IL-6) representing a key molecule.10 Patients with chronic HBV or HCV infection have higher IL-6 production than healthy controls and all 3 patients discussed herein were treated with anti–IL-6 therapy for CRS/CRES.11 Future studies will help to clarify the impact of chronic HBV/HCV infection on the risk of CRS and CRES.
Acknowledgment
The authors thank Jenny J. Kim at Kite, a Gilead Company, for medical review of 1 of the 3 presented cases, enrolled in the ZUMA-1 trial.
Authorship
Contribution: P.S. designed the study, analyzed data, and wrote the paper; L.J.N., L.E.F., F.S., and S.A. provided clinical care to patients; S.S.N. designed the study, analyzed the data, provided clinical care to patients, and wrote the paper; and all authors reviewed and approved the paper.
Conflict-of-interest disclosure: S.S.N. reports honoraria and research support from Kite, a Gilead Company, Merck, and Celgene; research support from Bristol-Myers Squibb, Poseida, Cellectis, Karus, and Acerta Pharma; and honoraria from Novartis, Pfizer, and Unum Therapeutics. F.S. reports honoraria from Celgene. L.J.N. reports honoraria from Celgene, Genentech, Gilead, Janssen, Juno, Novartis, and Spectrum and research support from Celgene, Genentech, Janssen, and Merck. The remaining authors declare no competing financial interests.
Correspondence: Sattva S. Neelapu, Division of Cancer Medicine, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: sneelapu@mdanderson.org.