In this issue of Blood, Parrow et al demonstrate that the 2 monoferric forms of transferrin (Tf), the major plasma glycoprotein involved in cellular iron delivery, have functionally distinct effects on erythropoiesis and systemic iron regulation.1
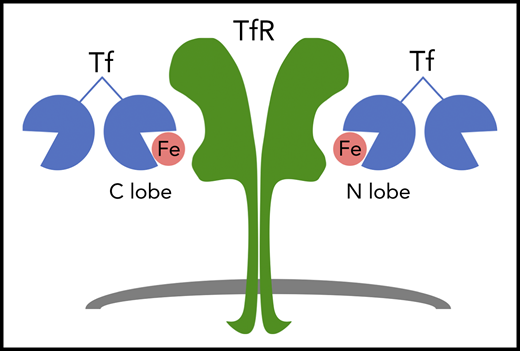
The plasma glycoprotein Tf binds up to 2 ferric (Fe3+) ions per molecule. Iron-bound forms of Tf bind to the Tf receptor (TfR) to deliver iron to cells. Tf is a bilobed protein that contains an iron binding site within each lobe. Thus, the monoferric forms of Tf carry iron in either their amino-terminal (N) lobe or carboxy-terminal (C) lobe.
The plasma glycoprotein Tf binds up to 2 ferric (Fe3+) ions per molecule. Iron-bound forms of Tf bind to the Tf receptor (TfR) to deliver iron to cells. Tf is a bilobed protein that contains an iron binding site within each lobe. Thus, the monoferric forms of Tf carry iron in either their amino-terminal (N) lobe or carboxy-terminal (C) lobe.
Synthesized by hepatocytes and secreted into the plasma, the glycoprotein Tf chaperones iron throughout the body. After binding to the Tf receptor (TfR1), iron-bound Tf is taken up by cells via clathrin-dependent receptor-mediated endocytosis, and iron is liberated from Tf within the cell.2 Tf is a bilobed, 80-kDa protein that binds one ferric (3+) ion per lobe with extremely high affinity (see figure).3 Thus, 4 distinct Tf species, which differ in the amount and/or distribution of bound iron, may circulate in the plasma. Because the diferric form of Tf has much higher affinity for the Tf receptor than monoferric Tf,4 diferric Tf is believed to be the major form of Tf that mediates iron delivery to erythroid cells. The specific contributions of the monoferric forms of Tf in mammalian physiology have remained unclear.
Parrow et al describe 2 novel transgenic mouse models in which the iron-binding capacity of Tf has been lost from the amino-terminal (N) lobe or the carboxy-terminal (C) lobe because of the mutation of specific tyrosine residues that normally coordinate iron. Mice homozygous for either the N-lobe–blocked or C-lobe–blocked form of Tf showed a reduction in the total iron-binding capacity of serum, as would be expected with a reduced number of iron-binding sites on Tf. In addition, both mutants showed an increase in the proportion of Tf iron-binding sites that were occupied by iron (ie, the percent Tf saturation). While maintaining serum iron levels within the normal range, mice homozygous for each Tf mutation displayed microcytic anemia that was accompanied by biochemical evidence of iron-restricted heme synthesis.
Intriguingly, although both Tf mutants were anemic, they exhibited differences in erythropoietic-related parameters. The C-lobe–blocked mutant showed a lower mean corpuscular volume and a higher red blood cell count, while the N-lobe–blocked mutant showed a lower hemoglobin level and a higher level of serum erythropoietin (EPO). Analysis of EPO-mediated events (ie, AKT phosphorylation, erythroferrone expression) suggested a relative impairment in erythropoietic responses to EPO in the N-lobe–blocked mutant compared with the C-lobe–blocked mutant. In addition, the N-lobe–blocked mutant failed to mount a hematologic response following exogenous EPO administration, whereas the C-lobe–blocked mutant showed a hematologic response to exogenous EPO that was even more robust than that of wild-type controls. Compared with wild-type controls, both Tf mutants developed hepatic iron overload, which was accompanied by relatively low serum levels of the iron regulatory hormone hepcidin. However, the N-lobe–blocked mutant showed lower serum hepcidin and higher liver iron content than the C-lobe–blocked mutant, suggesting differential effects on systemic iron regulatory mechanisms.
The study by Parrow et al demonstrates that monoferric forms of Tf are functionally important and mediate biologically distinct effects. The fact that both the N-lobe–blocked and the C-lobe–blocked Tf mouse mutants were viable and grew normally suggests that each monoferric form of Tf is capable of mediating iron delivery to tissues. Although monoferric forms of Tf deliver less iron than diferric Tf per each endocytic cycle, the anemia in the 2 monoferric Tf mutants indicates that their erythroid precursors are unable to upregulate TfR1 expression sufficiently to compensate and maintain normal erythropoiesis.
The Parrow et al study also raises a number of questions about the underlying mechanisms driving the erythropoietic and hepcidin responses observed in the 2 Tf mutants. For example, could their phenotypic differences be explained by differing affinities of the monoferric forms of Tf for Tf receptors in vivo, or alternatively, by differing effects of each bound form of monoferric Tf on Tf receptor function? Notably, in addition to serving as a ligand for TfR1, Tf also binds the homologous receptor TfR2, which has a much lower binding affinity for Tf and has thus been proposed as a sensor of alterations in Tf saturation.5 In contrast to the broad tissue expression of TfR1, the expression of TfR2 seems to be restricted to a smaller set of tissues, including erythroid progenitors and hepatocytes. Although TfR1 mediates iron uptake by erythroid cells, TfR2 is important for the stability of the EPO receptor and seems to modulate the response to EPO according to iron availability.6 In the liver, TfR2 plays a critical role in regulating hepcidin production, because its loss results in a hemochromatosis phenotype characterized by hepcidin deficiency and liver iron loading.5 By contrast, loss of TfR1 in hepatocytes results in low hepatic iron content that is accompanied by an inappropriate elevation of hepcidin.7
The Tf receptor (TfR1) functions as a homodimeric transmembrane protein in which each TfR1 monomer binds 1 Tf molecule in its ectodomain. Structural studies indicate that the N- and C-lobes of Tf have distinct sites of physical contact with TfR1, such that the C-lobe interacts with the side of the TfR1 homodimer, whereas the N-lobe resides between the TfR1 ectodomains and the cell membrane.8,9 It is possible that the interactions of TfR1 (or TfR2) with other proteins, such as the hemochromatosis protein HFE, may be differentially modulated by each monoferric form of Tf, thereby having an impact on downstream signaling events. Whether specific phenotypes observed in the N-lobe and C-lobe Tf mutants can be attributed to the presence of iron in a particular lobe or the absence of iron from the alternative lobe is not yet clear.
In terms of clinical relevance, the findings of Parrow et al raise the possibility that quantification of the different iron-bound forms of Tf might have value in clinical settings associated with EPO resistance, whereas administration of the C-lobe–blocked form of Tf might provide a means to increase EPO sensitivity. Of note, a relative shift toward monoferric forms of Tf is expected in hypoferremic states. Thus, an improved understanding of mechanisms by which the monoferric forms of Tf impact erythropoiesis and systemic iron regulation may provide physiological insights that are particularly relevant to clinical settings such as iron deficiency anemia and the anemia of inflammation.
Conflict-of-interest disclosure: K.E.F. declares no competing financial interests.