Introduction: The management of immune thrombocytopenia (ITP) in pregnancy can be challenging as some patients either do not respond to or tolerate corticosteroids and intravenous immunoglobulin and only very few alternative ITP therapies are available during pregnancy. The use of thrombopoietin receptor agonists (Tpo-RA) which are likely to cross the placenta is not recommended during pregnancy but both romiplostim and eltrombopag have been exceptionally used to treat women with severe and refractory ITP during pregnancy. To better assess safety and efficacy of Tpo-RA during pregnancy, we performed an international multicentre observational retrospective study.
Methods: To be included, the patients had to fulfill the following criteria: pregnant woman aged of 18 years and above, diagnosis of primary or secondary ITP according to international consensus guidelines, use of either eltrombopag of romiplostim for at least 1 week for treating ITP during pregnancy (before delivery), at least a month of follow-up after Tpo-RA initiation. Women who became pregnant while on Tpo-RA could be included even if the treatment was stopped if enough data on pregnancy outcome were available. Women treated with a Tpo-RA during pregnancy not for ITP were excluded. All clinical and biological data were collected by means of a standardized study form, whenever available, data on the neonates were also collected and analyzed. Data are presented as mean±SD or median (interquartile range [IQR]) for continuous variables, depending on their distribution. Categorical variables are presented as number (%).
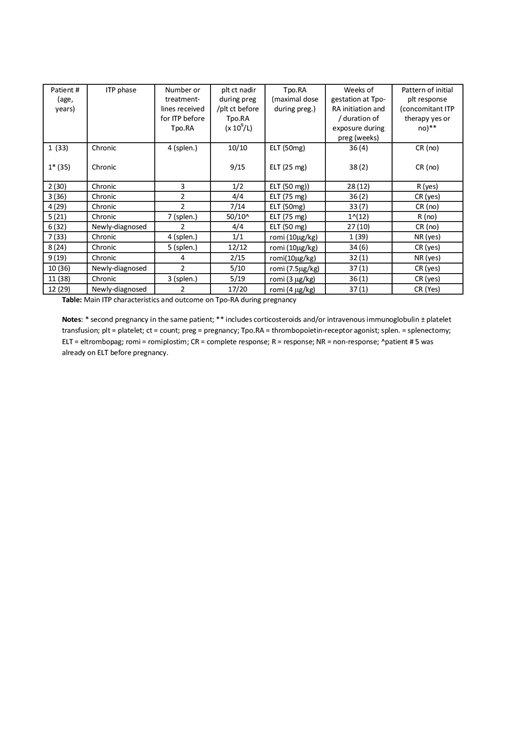
Results: In total, 12 women (mean age at time of pregnancy was 30.3 ± 5 years) fulfilling the eligibility criteria were included, for a total of 13 pregnancies and 14 neonates (one twin pregnancy) with an exposure to Tpo-RA. Nine of 12 patients had pre-existing chronic primary ITP (mean ITP duration = 11.8 ± 10.1 years) whereas ITP was newly-diagnosed during pregnancy in 3 cases. The median number of treatment-lines before the use of Tpo-RA was 3 [range 2-7] including splenectomy for 5 patients. Patients were treated transiently during pregnancy with either eltrombopag (n = 6; mean daily dose 50mg) or romiplostim (n = 6; mean maximal weekly dose 7.4 microg/kg). Two patients with chronic ITP were already on Tpo-RA when pregnancy was confirmed, and for 8 pregnancies, treatment with Tpo-RA was initiated only within 4 weeks before term in preparation for delivery. The median time of exposure to Tpo-RA during pregnancy was 4.4 weeks [range: 1-12 weeks]. No side-effects and especially no thromboembolic events were observed; none of the patients was on thromboprophylaxis. The mean platelet count at term was 91 x 109/L (median = 94 x 109/L [6-250]). Delivery occurred pre-term in 4 out of 13 pregnancies, mode of delivery was vaginal in 8 out of 13 pregnancies (with an epidural in 4 cases) and a C-section in 5. The platelet count was available at birth in 10 out of 13 neonates and neonatal thrombocytopenia was found in 5 (including 3 from the same mother). No case of neonatal thrombocytosis was observed. No neonatal complications attributable to the exposure to a TpoRA in the mother was observed. One neonate (whom the mother received 1 week of romiplostim in preparation for delivery) was diagnosed with trisomy 8 and died on day 7 and another neonate had a pulmonary artery stenosis diagnosed during fetal life (before the initiation or Tpo-RA in the mother), that was successfully operated at 2 weeks of life. A complete platelet response (CR) was achieved on Tpo-RA during pregnancy in 8/12 patients (66%) (5 of them received concomitant ITP therapy), a response (R) in 2 whereas no response was achieved in 2 patients with refractory ITP (table).
Conclusion: Based on this preliminary results on a relatively small number of patients (more cases are expected) and taking into account that Tpo-RA was used only in preparation for delivery in 7/13 pregnancies, a temporary off-label use of a Tpo-RA over a short period of time for ITP during pregnancy seems safe for the mother and the neonate. The pattern and magnitude of response seems comparable to what is observed outside pregnancy but only few patients were treated with Tpo-RA alone. For now, the transient use of Tpo-RA during pregnancy should only be considered exceptionally for women with severe and refractory ITP.
Michel:Rigel: Consultancy; Amgen: Consultancy; Novartis: Consultancy. Ghanima:Amgen: Consultancy, Honoraria; Bayer: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer/BMS: Research Funding. Anderson Tvedt:Alexion: Other: Advisory Board; Ablynx: Other: Advisory Board; Novartis: Other: Advisory Board. Bussel:Tranquil: Honoraria, Membership on an entity's Board of Directors or advisory committees; Physician Education Resource: Speakers Bureau; Kezar Life Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; argenx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; 3S Bio: Speakers Bureau; Rigel: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Dova Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Momenta Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; RallyBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Godeau:Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau.
It reports some data about the use of either romiplostim or eltrombopag (thrombopoietin receptor agonists) to treat ITP during pregnancy. Both drugs are licensed for adult' ITP but are not supposed to be used in pregnant women
Author notes
Asterisk with author names denotes non-ASH members.