Introduction: Clinical cytogenetics is the most important prognostic test for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS); however, current tools do not provide a complete genome-wide picture of somatic chromosomal aberrations in those patients. We used a high-resolution genome-wide single-nucleotide polymorphism (SNP) array to identify large clonal chromosomal aberrations in pre-hematopoietic cell transplant (HCT) peripheral blood samples of patients with de novo or therapy-related AML and MDS.
Methods: We used the HumanOmniExpress-24 BeadChip SNP array genotyping data generated in the DISCOVeRY-BMT study (includes patients with acute leukemia or MDS who received unrelated donor HCT between 2000-2011). Clinical data and biospecimens were obtained from the Center for International Blood and Marrow Transplant Research. Blood samples were collected at median of 7 days before HCT. We limited our analysis to patients with AML (N=1,982) and MDS (N=608). We calculated the log2 R ratio and B allele frequency (BAF) and used the Circular Binary Segmentation (SBC) algorithm to identify chromosomal aberrations spanning ≥2Mb.
Results: Median age at sample collection was 48.2 (range=0.6-78.0) and 53.0 (range=0.0-74.0) years for AML and MDS, respectively. About 53% of AML and 59% of MDS patients were males, and 7.4% of AML and 15.8% of MDS patients had therapy-related disease. Among AML patients, 47.9% were in 1st complete remission and 22.8% in 2nd complete remission. For MDS, 56.1% of patients were in early stage (refractory anemia with or without ringed sideroblasts) at the time of HCT.
Chromosomal aberrations were detected in 14.6% of the AML patients (n=289) and 34.7% of the MDS patients (n=211). In AML, 6.6% of those with hematological or molecular remission vs. 35.9% of those with advanced disease (not in remission) had SNP-array detected aberrations, with 0.7% of the patients vs. 8.9%, respectively carrying aberrations in ≥3 chromosomes. For MDS, chromosomal aberrations were detected in 31.4 vs. 39.0% of patients with early and advanced MDS, respectively. No differences by patient sex or race were detected in both AML and MDS (p>0.05).
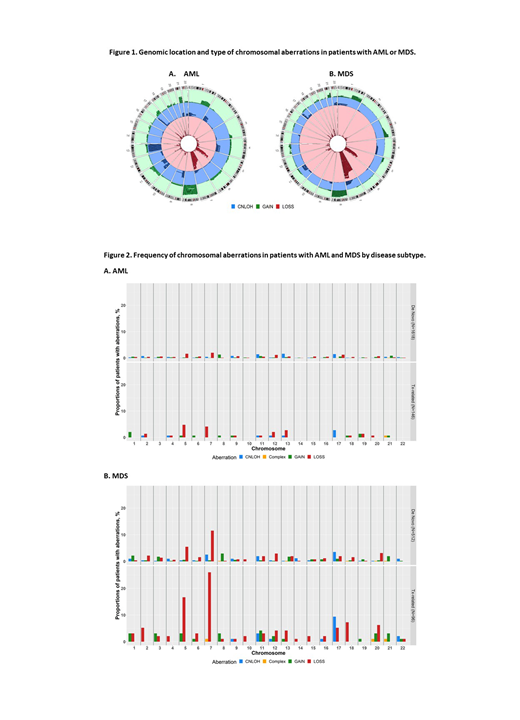
Figure 1 shows disease-specific type and frequency of detected chromosomal aberrations. Copy-losses in chr7 (chr7-), chr5 (chr5-), and copy-neutral loss of heterozygosity (CNLOH) in chromosome 17 (chr17-CNLOH) were the most common aberrations in both diseases, with higher frequencies in MDS (chr7-, 13.8 vs. 2.4%, respectively; chr5-, 7.2 vs. 1.8%; chr17-CNLOH, 4.4 vs. 1.5%, respectively, all p<0.0001). In comparison, CNLOH in chr13 was more frequent in AML than MDS (1.5 vs. 0.2%, respectively, p<0.01).
Figure 2A shows detected chromosomal aberrations in de novo and therapy-related AML (tAML). The following were more frequently detected in tAML than de novo disease: copy-losses of chr5 (4.8 vs. 1.6%, p=0.02) and chr13 (2.7 vs. 0.6%, p=0.02). Aberrations in chromosomes 3, 10, 14, 15, 16 and 22 were found in de novo but not in tAML. The frequencies of chr7- (4.1 vs. 2.0%, in tAML and de novo, respectively, p=0.12) and copy-gain in chr8 (0.7 vs. 1.3%, p>0.99) were similar in the two disease subtypes.
Figure 2B shows detected chromosomal aberrations in de novo and treatment-related MDS (tMDS). The following were more common in tMDS than de Novo MDS: copy losses in chr5 (16.7 vs. 5.5%, p<0.001), chr7 (26.0 vs. 11.5%, p<0.001), and chr18 (7.3 vs. 1.6%, p<0.01); also copy-gain in chr11 (4.2 vs. 0.4%, p<0.01), and chr17-CNLOH (9.4 vs. 3.5%, p=0.03). Unique aberrations in de novo MDS included CNLOHs in chr7 (2.5%), chr14 (1.2%), chr1 (1.0%), chr4 (1.0%) and copy-gain in chr17 (1.0%).
Conclusion: High-resolution SNP array analysis provided a genome-wide landscape of large chromosomal aberrations in patients with AML and MDS. Detected aberrations were more frequent in MDS than AML patients; this is possibly affected by differences in pre-HCT initial therapy. This study showed the expected genomic landscape similarities between AML and MDS, but differences were also present (most noted was chr13-CNLOH predominance in AML). The observed differences between de novo and t-AML or t-MDS likely reflect distinct pathogenic processes. Differences between t-AML and t-MDS could be related to antecedent malignancy and/or therapeutic regimen. The impact of these detected aberrations on HCT outcomes is under investigation.
Griffiths:Novartis Inc.: Consultancy; Celgene, Inc: Consultancy, Research Funding; Celgene, Inc: Consultancy, Research Funding; New Link Genetics: Consultancy; New Link Genetics: Consultancy; Persimmune: Consultancy; Boston Scientific: Consultancy; Boston Scientific: Consultancy; Persimmune: Consultancy; Genentech, Inc.: Research Funding; Abbvie, Inc.: Consultancy, PI on a clinical trial; Astex Phramaceuticals/Otsuka Pharmaceuticals: Consultancy, Research Funding; Abbvie, Inc.: Consultancy; Astex Phramaceuticals/Otsuka Pharmaceuticals: Consultancy, Research Funding; Genentech, Inc.: Research Funding; Novartis Inc.: Consultancy; Appelis Pharmaceuticals: Other: PI on a clinical trial; Onconova Therapeutics: Other: PI on a clinical trial; Appelis Pharmaceuticals: Other: PI on a clinical trial; Onconova Therapeutics: Other: PI on a clinical trial; Partner Therapeutics: Consultancy; Partner Therapeutics: Consultancy. Thota:Incyte, Inc.: Speakers Bureau. Pasquini:Novartis: Research Funding; Kit Pharma: Research Funding; BMS: Research Funding; Medigene: Consultancy; Amgen: Consultancy; Pfizer: Other: Advisory Board. Lee:Takeda: Research Funding; Novartis: Research Funding; Amgen: Research Funding; Syndax: Research Funding; Incyte: Research Funding; Kadmon: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; AstraZeneca: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.