Background
8q24 translocations leading to overexpression of MYC are an established prognostic marker in multiple myeloma (MM). Currently FISH (fluorescence in situ hybridization) on CD138+ enriched cell population is the standard diagnostic approach to evaluate the presence of 8q24 translocations. Due to the heterogeneity of breakpoints and technical issues the design of FISH probes is challenging and so far no single FISH assay is capable of detecting each translocation.
Aims
(1) Evaluation of the frequency of 8q24 translocations in MM by whole genome sequencing (WGS). (2) Determination of the breakpoints on 8q24 and partners. (3) Correlation of WGS data with FISH and MYC expression determined by whole transcriptome sequencing (WTS).
Patient cohort and methods
CD138+ cell fractions were selected by MACS from bone marrow aspirate samples of 264 patients diagnosed with MM. FISH, WGS and WTS were performed in all cases. For WGS, 151bp paired-end sequences where generated on NovaSeq 6000 machines (Illumina, San Diego, CA). All reported p-values are two-sided and were considered significant at p<0.05. For gene expression (GE) analysis by WTS, estimated gene counts were normalized and the resulting log2 counts per million were used as a proxy of gene expression in each sample. For artefact exclusion, structural variants were checked against 4386 cases covering the spectrum of hematological malignancies.
Results
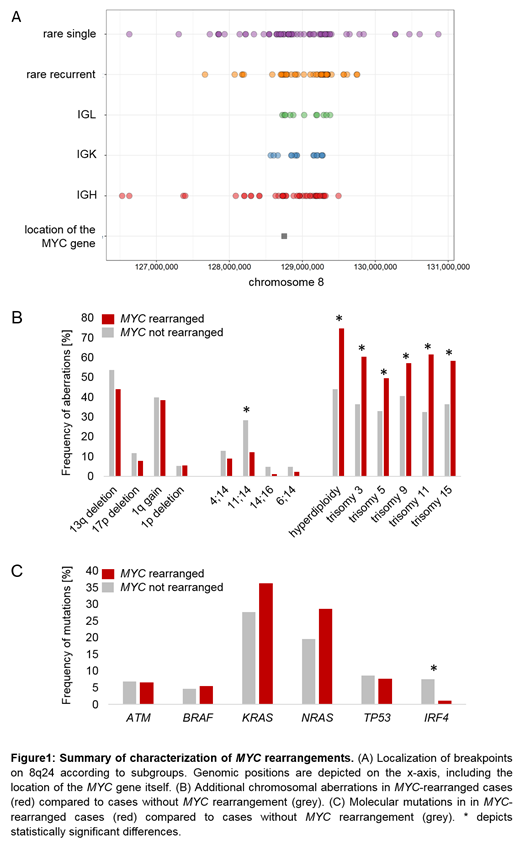
In 91/264 (34%) of cases, at least one rearrangement involving the MYC locus (MYCr) was detected by WGS. In 18 of these samples (20%), >1 MYCr was present (114 MYCr in total). Out of these 91 patients, in 32 (35%) the MYCr had been identified by FISH, in 46 cases (51%) it was not detected due to the heterogeneity of breakpoints, while in 13 (14%) patients FISH could not be evaluated (e.g. due to insufficient patient material). Of the 114 MYCr encountered in WGS, 42 involved one of the immunoglobulin loci (IGH n=25, IGK n=9, IGL n=8). The remaining 72 MYCr involved other rare partners. In 29 of these rearrangements, as well as in four complex rearrangements involving IGH or IGK, recurrent rare partners were identified, comprising 1p12/FAM46C (n=6), 6p24.3/BMP6 (n=10), 6q21/FOXO3 (n=4), 7p21.3 (n=3), 11q13/CCND1 (n=5), 20q11.22 (n=5). 43 MYCr involved non-recurrent (single) rare partners, for 4 of these a MYCr was also detected by FISH. The MYCr detected were rather complex: only 34 (30%) showed a simple reciprocal translocation (IGH n=7, IGL n=2, IGK n=4, rare partners n=21), 60 (53%) showed more complex rearrangements (IGH n=12, IGL n=4, IGK n=2, rare partners n=42) and in 20 cases (18%) at least one additional chromosome was involved (IGH n=6, IGL n=3, IGK n=2, rare partners n=9). In 80% of MYCr, breakpoints were located between genomic positions 128.203.605 and 129.375.490 encompassing the pre-described MYC surrounding locus PVT1. IGH-MYC rearrangements showed a tendency to cluster towards the centromere. MYCr involving rare partners showed the broadest breakpoint spectrum and clustered in both directions of the hotspot (Fig 1A). Regarding expression of MYC, all cases showed an overexpression (median GE: 6.9 vs 4.5 in normal controls). Median GE was similar in cases with Ig partners (IGH: 7.1, IGL: 6.7, IGK: 6.6) and non Ig partners (6.8) and also in cases with MYCr detected by FISH (7.0) and cases in which it was not detected by FISH (6.5). Analysis of additional chromosomal aberrations revealed that hyperdiploidy was significantly more frequent in MYCr (n=68/91, 75% vs n=76/173, 44%; p=0.001), while t(11;14) was found significantly less frequent (n=11/91, 12% vs n=49/173, 28%; p=0.003) (Fig 1B). No associations were found between MYCr and other frequent chromosomal abnormalities. Furthermore, molecular mutations frequently occurring in MM (ATM, BRAF, KRAS, NRAS, TP53, IRF4) were analyzed, revealing that patients with MYCr were significantly less frequently associated with mutations in the IRF4 gene (MYCr patients n=1/91; non-MYCr patients n=13/173; p =0.028) (Fig 1C).
Conclusions
(1) WGS detects ~3x more MYCr compared to FISH. (2) The complexity on the genomic level of MYCr is high, therefore the detection with targeted assays is limited while WGS allows a more comprehensive analysis. (3) MYC expression in cases with MYCr with non Ig partners is comparably high as for Ig-MYC translocations. (4) MYCr are associated with hyperdiploidy, whereas t(11;14) and IRF4 mutations were detected at a lower frequency.
Seliger:MLL Munich Leukemia Laboratory: Employment. Geirhos:MLL Munich Leukemia Laboratory: Employment. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Kern:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Walter:MLL Munich Leukemia Laboratory: Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Employment. Baer:MLL Munich Leukemia Laboratory: Employment. Stengel:MLL Munich Leukemia Laboratory: Employment. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.