Introduction: Daratumumab (DARA) is an anti-CD38 human monoclonal antibody FDA-approved for the treatment of multiple myeloma (MM) and has demonstrated efficacy in AL amyloidosis. Infusion-related reactions (IRRs) occur in over 50% of patients treated with DARA, most commonly with the first infusion despite premedication with corticosteroids, antipyretics, antihistamines, and post-infusion corticosteroids. If DARA is tolerated without IRRs, the infusion rate can be escalated in standardized increments to a final infusion rate administered over 90 minutes in patients with MM (Barr et al. Leukemia 2018). Rapid infusions of DARA decrease length of infusion visits, potentially improving patient satisfaction and optimizing healthcare resources. Recent studies showed that DARA is safe and highly effective in treating AL amyloidosis (Khouri et al, Br J Haematol 2018), but no information is available regarding the safety of administering rapid infusions of DARA in patients with AL amyloidosis. We hypothesized that patients with AL amyloidosis, especially those with cardiac involvement, may not tolerate a rapid administration of fluids. Thus, a DARA rapid infusion protocol for patients with AL amyloidosis was implemented at our institution in April 2018. We report findings from the protocol implementation.
Methods: A retrospective observational study was conducted to review adult patients with AL amyloidosis who received rapid infusions of DARA from April 2018 to October 2018. Data collected included relevant past medical history, vitals, premedications, infusion rates, and management of IRRs. Patients were eligible for rapid DARA after tolerating two prior doses of DARA at standard infusion rates. Rapid DARA was infused at 200 mL/hr for 30 minutes, then increased to 450 mL/hr for 60 minutes. Protocol premedications included acetaminophen, diphenhydramine, famotidine, and dexamethasone. No post-infusion corticosteroids were required. The first two standard and first four rapid DARA infusions were evaluated for each patient.
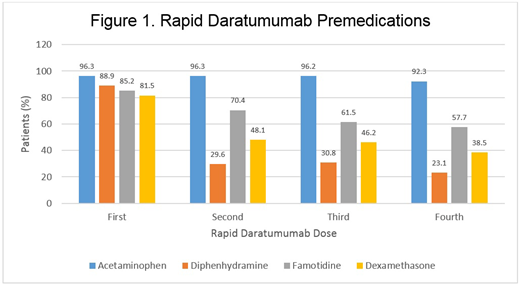
Results: 27 patients with AL amyloidosis were included in the study with 162 doses of rapid DARA evaluated. Baseline characteristics included age (median 72 years old), gender (55.6% male), diastolic heart failure (59.3%), combined systolic and diastolic heart failures (11.1%), chronic obstructive pulmonary disease (7.4%), asthma (7.4%), and chronic kidney disease (25.9%). 62.9% of patients had cardiac AL involvement and 51.9% had renal AL involvement. 11/27 (40.7%) patients had IRRs to the first infusion of DARA (cycle 1, day 1) requiring hypersensitivity medications. All 27 patients progressed to rapid rate DARA after both initial and subsequent rates were tolerated. 22/27 (81.5%) patients initiated the first rapid DARA with the protocol premedications. Premedication usage decreased by the fourth rapid DARA dose with only 23.1% patients receiving diphenhydramine, 57.7% receiving famotidine, and 38.5% receiving dexamethasone (Figure 1). No patients received post-infusion corticosteroids. There were no clinically significant IRRs with the DARA rapid infusion protocol across all patients, regardless of organ involvement. There were 10 cases of CTCAE grade 2-3 hypertension that did not require intervention. No hypersensitivity medications were required for any rapid DARA doses. 11/11 (100%) patients who experienced IRRs requiring hypersensitivity medications during their first or second standard DARA infusions tolerated rapid DARA administration without recurrence of IRRs.
Conclusions: Rapid administration of DARA is safe and well-tolerated in patients with AL amyloidosis, regardless of organ involvement. Patients who experienced an IRR with the initial DARA dose were able to safely transition to the rapid infusion protocol without recurrence of IRRs. Furthermore, decreased premedication usage after tolerance of rapid DARA infusion did not increase IRRs, suggesting premedications can be discontinued to mitigate associated toxicities.
Anwer:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; In-Cyte: Speakers Bureau. Valent:Celgene corporation: Speakers Bureau; Amgen corporation: Speakers Bureau; Takeda pharmaceuticals: Speakers Bureau.
Daratumumab for the treatment of AL amyloidosis
Author notes
Asterisk with author names denotes non-ASH members.