Lenalidomide in combination with dex (Len + dex) was introduced as treatment for relapsed/refractory myeloma in Canada over a decade ago; more recently, Len has been routinely available as part of first-line therapy, both in transplant-eligible patients-- as post-ASCT maintenance--and in transplant-ineligible patients as the Len + dex combination, and is typically given until disease progression. The management of patients progressing on Len regimens is evolving as newer anti-myeloma drugs become available. Many questions remain regarding the sequencing of treatments to obtain the longest periods of disease control between repeated relapses. We utilized the national Myeloma Canada Research Network (MCRN) Database to analyze therapy administered immediately after progression on a Len-based regimen in real-world practice.
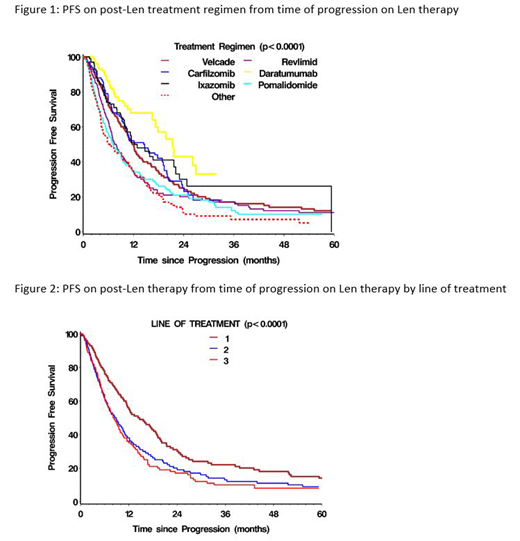
The MCRN Database contains disease-specific information on over 6000 patients reported from 13 academic centres in Canada. Between 2007 and 2019, 1482 patients (pts) progressed on Len-containing regimens in lines 1-3. 57% were male, 23% had light chain myeloma, 38% had high-risk FISH and 43% had undergone prior ASCT. Median values (range) for other pt characteristics included: age 64 range (31-92) yrs, creatinine 93 µmol/L (32-2700), B2M 329 nmol/L (range 1-7193), Hb 104 g/L (3-169), WBC 5.7 (0.12-107.6), platelets 211 (10-832), LDH 191 U/L (55-1908). The next regimen was based on bortezomib (BTZ) in 370, as part of a triplet in 278 (CyBorD, VMP); carfilzomib (CFZ) in 100, triplet in 62(KCD, KRD, KPD); ixazomib (IXA) in 75, triplet in 63 (IxaCD, IxaRD, IxaPomD); daratumumab (Dara) in 80, triplet in 75 (DaraCD, DaraRD, DaraPomD, DaraVD, DaraKD); pomalidomide (POM) in 195, triplet in 79 (PomCD/P, PomVD, PomKD); and continuation of Len in 212, triplet in 124 (RCD, RVD, RVCD). 346 (23%) did not receive any further therapy, including 79 (15%) after 1st line Len, 199 (26%) after 2nd line Len and 68 (33%) after 3rd line Len; other regimens were used in 6%, 18% and 11% of each group, respectively. The overall response rates (ORR)/median duration of treatment in months (mos) for each of the regimens included: BTZ 67%-5.8 mos; CFZ 70%-5.6 mos; IXA 60%-6.7 mos; Dara 86%-6.9 mos; POM 36%-4.0 mos; Len 39%-5.7 mos; and other 48%-3.3 mos. PFS by regimen is shown in Figure 1.
514 pts had progressed after Len as 1st line therapy, 766 after Len as 2nd line therapy and 202 after Len as 3rd line therapy. Median follow-up was 15 mos (1-130) after 1st progression and 55 mos (1-278) from diagnosis. The median PFS (95% CI) and OS (95% CI) after progression following Len as 1st line therapy were 14.5 mos (12.1-17.3) and 31.2 mos (25.3-39.0), as 2nd line therapy 8.6 mos (7.3-9.8) and 14.1 mos (11.6-16.5); and as 3rd line therapy 8.0 mos (6.7-9.9) and 11.0 mos (6.9-14.1), respectively. Figure 2 shows PFS by prior Len subgroup. More detailed analyses to assess outcomes for specific regimens after Len progression in different lines of therapy are in progress.
In summary, this analysis provides an overview of treatment patterns following progression on Len in Canada, as well as the ORR, PFS and OS of different regimens. Results appeared better for proteasome inhibitor and Dara regimens after progression on Len, although some pts derived benefit from further Len regimens as well as POM-based ones; further exploration of specific treatment subgroups is ongoing. These data provide benchmarks for real-world outcomes that can be utilized as newer therapies, such as those based on immunotherapy, become available.
Reece:Amgen: Consultancy, Honoraria, Research Funding; Merck: Research Funding; BMS: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. McCurdy:Janssen: Honoraria; Celgene: Honoraria. Song:Janssen: Honoraria; Celgene: Honoraria, Research Funding; Amgen: Honoraria; Takeda: Honoraria. Sebag:Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Leblanc:Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Louzada:Celgene: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Bayer: Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. White:Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Stakiw:Janssen: Honoraria, Research Funding, Speakers Bureau; Roche: Research Funding; BMS: Honoraria; Novartis: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Lundbeck: Honoraria; Sanofi: Honoraria. Kotb:Karyopharm: Equity Ownership; Amgen: Honoraria; Merck: Honoraria, Research Funding; Celgene: Honoraria; Janssen: Honoraria; Takeda: Honoraria. Venner:J&J: Research Funding; Takeda: Honoraria; Sanofi: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria, Research Funding.
This abstract describes several combinations not specifically approved by the FDA but utilized in the real-world setting. However, all of the individual drugs in these combinations have been approved as single agents or in other combinations.
Author notes
Asterisk with author names denotes non-ASH members.