Although life extending medical treatments are available for sickle cell disease (SCD), allogenic hematopoietic stem cell transplantation (HSCT) is considered a treatment of choice (Ozdogu et al., Bone Marrow Transplant 2018; 53(7): 880-890). However, HSCT is associated with several limitations, including conditioning-related toxicity and graft-versus-host disease (GvHD), especially when using MHC disparate transplants. Thus, the development of safe transplantation protocols for MHC disparate HSCT in sickle cell disease is vital. While the risk of GvHD and conditioning toxicity can be effectively reduced by the use of T-cell-depleted HSCT (TD-HSCT) under reduced intensity conditioning (RIC), rejection of TD-HSCT remains a challenge. We previously demonstrated in wild type mice that this barrier can be overcome using donor-derived veto cells. Here, we demonstrate the safety and efficacy of this approach in a well-defined murine model for sickle cells disease.
Veto activity is based on the ability of certain cells to attack host CTL-precursors (CTLp) which are directed against antigens expressed on the veto cells themselves, sparing cells that are not targeted against the veto cells including T cells needed for defense against pathogens. Central memory CD8 T cells exhibit the most robust veto activity upon transplantation; however, these cells are also endowed with marked GvH activity. We overcame this issue by expanding naïve or memory CD8 T cells against 3rd party MHC or viral antigens, respectively, under culture conditions favoring expression of central memory phenotype. Such anti-3rd party central memoryCD8 T cells (Tcm), which are endowed with marked veto activity, also exhibit reduced risk for GvHD in fully mis-matched recipients (Reviewed in Reisner Y, Or-Geva N. Semin Hematol. 2019; 56(3): 173-182.)
To generate Tcm veto cells, splenocytes obtained from Balb/c donors (H2d) were cultured against irradiated third-party splenocytes (FVB; H2q) under cytokine deprivation. The selective expansion of CD8 mouse T cells against 3rd party stimulators leads to selective 'death by neglect' of bystander anti-host T cell clones potentialy mediating GvHD, and these are further diluted out by subsequent expansion of anti-3rd party T clones during continued culture in the presence of IL-15. Apart from selective loss of GvH reactive T cells, these culture conditions induce a central memory phenotype shown to be important for attaining robust veto activity in vivo (Ophir et al., Blood. 2013; 121(7): 1220-8).
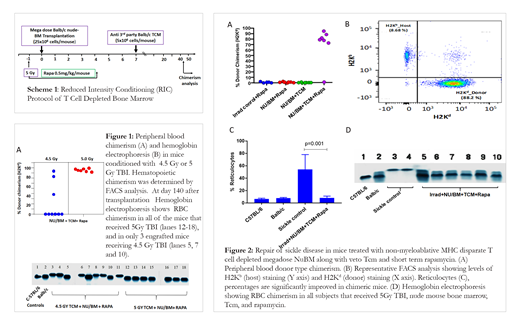
We first calibrated the optimal irradiation dose for sickle mice (Berkeley model, H2b), comparing 4.5 Gy versus 5 Gy TBI in a conditioning protocol also including short term rapmycin treatment (Scheme 1). Higher levels of engraftment and chimerism were found in the group receiving 5Gy TBI (Fig. 1). All mice of both treatment groups survived >140 days with no evidence of GvHD. To further evaluate this treatment approach, 8 week old sickle mice (N=7) were given bone marrow transplants using the protocol described in Scheme 1, including conditioning with 5 Gy TBI (day -1), rapamycin treatment (day -1 to day 4), and transplantation of NuBM (day 0) plus veto cells (day 7; Scheme 1). Notably, at 44 days post-transplant, 6 out of 7 mice receiving NuBM + TCM + Rapa (85.7%) showed donor chimerism in the peripheral blood, ranging between 77-94% (Fig.2A-B), while no chimerism was detected in mice receiving conditioning alone, or conditioning and transplantation with only NuBM or only veto cells . All mice in all groups survived (N=26) , and no GvHD was detected with a follow up of 77 days, even in the transplanted group which exhibited high donor-derived chimerism. Furthermore, reversal of sickle disease symptoms was observed, including reticulocyte levels (p=0.001;Fig.2C) and expression of wild type hemoglobin ( Fig.2D) in all engrafted mice.
Our results offer a proof of concept for the treatment of sickle disease by MHC disparate non-myeloablative T cell depleted HSCT in conjunction with anti-3rd party central memory veto CD8 T cells. A clinical trial testing the safety and efficacy of anti-3rd party veto cells in the context of low toxicity non-myeloablative TD-HSCT in hematological malignancies is currently ongoing at MD Anderson Cancer Center. If successful, our present results support further evaluation of this platform for sickle cell disease.
Lustig:Yeda Ltd.: Patents & Royalties. Reisner:Cell Source, Inc.: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.