Post-transplant cyclophosphamide (PTCy) based GVHD prevention regimens are an emerging platform in allogeneic transplantation for hematological malignancies and are being prospectively compared to traditional standards. We present a single-center retrospective series of adult allogeneic bone marrow transplantation with the use of post-transplant cyclophosphamide in the setting of benign hematological conditions. Ten patients were treated between 2013 to 2019. Non-myeloablative conditioning consisted of rATG 0.5mg/kg on day (D) -9, 2mg/kg on D-7,-8 (ATG was in one patient with DBA), fludarabine 30mg/m2 daily from D-6 to D-2, cyclophosphamide 14.5mg/kg D-6 and D-5, and 200cGy of total body irradiation D-1. The bone marrow graft was administered on D0. GVHD prophylaxis consisted of post-transplant cyclophosphamide at 50mg/kg/day IV D+3, and +4 mycophenolate mofetil (MMF) at 15 mg/kg 3 times daily (maximum daily dose 3000 mg) starting on D+5 with taper beginning at D+35 to be off around D+100, and tacrolimus on D+5 with a target trough of 5-10 ng/mL with tapering beginning at D+180 with a goal to be off at D+360 in the absence of any GVHD.
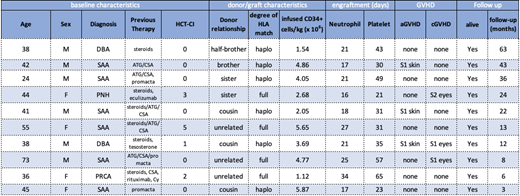
The median age at the time of transplantation was 42 (range 24-73) and 4 of 10 were female. Diagnoses include severe aplastic anemia (n=6), Diamond-Blackfan Anemia (n=2), paroxysmal nocturnal hemoglobinuria (n=1), and pure red cell aplasia (n=1). Median Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) was 0 (range 0-5). Bone marrow donors were haploidentical donors (n=6), fully matched unrelated donors(n=3), and fully matched sibling (n=1). The median donor age was 31 (range 22-53). Donor marrow grafts had a median CD34+ cell count of 4.41 x 106/kg recipient ideal body weight (range 1.12 x 106 to 20.5 x 106).
At a median follow-up of 17 months (range 3, 63) after transplantation, all patients are alive, with donor-derived hematopoiesis and free of significant acute or chronic GVHD. Neutrophil and platelet engraftment occurred at a median of 21 (range 16-34) days and 33 (21-65) days, respectively, after transplantation. The patient with pure red cell aplasia developed secondary graft failure and required a second transplant using a peripherally collected graft with the same fully matched unrelated donor. For all recipients, median day +30 unsorted chimerism was 100% (range 71-100%). Patients have not experienced significant acute or chronic GVHD. There were no cases of grade II-IV acute GVHD observed. Chronic GVHD was observed in 3 patients with ocular disease (two mild, one moderate). All patients who are over 12 months after transplantation are off systemic immunosuppression.
Three patients experienced CMV reactivation, two required preemptive treatment with oral valganciclovir, while the third had a single positive low level positive CMV PCR that resolved spontaneously. Three patients had low level positive EBV viremia, none of which required pre-emptive treatment. Other significant infectious complications before day +100 included BK cystitis in three patients, influenza, adenovirus cystitis, clostridium difficile colitis, streptococcal and enterococcal polymicrobial endocarditis, and enterococcal bacteremia. Significant non-infectious complications were rare. One patient experienced engraftment syndrome which resolved quickly with systemic steroid administration. Another patient suffered a small spontaneous subdural hemorrhage day +10 after transplantation, but subsequently made a full neurologic recovery.
Post-transplant cyclophosphamide based non-myeloablative allogeneic bone marrow transplantation appears safe and effective for patients with non-malignant hematologic conditions and should be prospectively compared to historical regimens.
Table 1. Cohort Characteristics. SAA - Severe aplastic anemia, DBA - Diamond Blackfan anemia, ATG- anti-thymocyte globulin, CSA- cyclosporine, Cy-cyclophosphamide, haplo- haploidentical
Defilipp:Incyte: Research Funding. Frigault:Nkarta: Consultancy; Novartis: Consultancy; Foundation Medicine: Consultancy; Xenetic: Consultancy; Juno/Celgene: Consultancy; Kite/Gilead: Honoraria; Incyte: Consultancy. Chen:Incyte: Consultancy; Magenta: Consultancy; Takeda: Consultancy; Kiadis: Consultancy; Abbvie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.