Introduction: Prophylactic T cell depletion with antithymocyte globulin (ATG) remains a standard of care for GVHD prophylaxis during allotransplant (ASCT). Although the optimal ATG dosing strategy is still unknown, recent studies have reported that recipient absolute lymphocyte counts (ALC) at the time of ATG administration may predict survivals in ASCT with unrelated donors, suggesting that the dose (especially at the cut off of <0.1x109/L) and timing of ATG administration must be taken into account (Soiffer et al, JCO 2017; Kennedy et al, BBMT 2018). Our experience on the impact of lymphopenia at the time of ATG administration during allotransplant is reported here.
Materials & Methods: All adults transplanted in our department between 01/2009 and 03/2019 with a Purine analogue/Busulfan/ATG based conditioning regimen and PBSC as source of graft from a matched or 9/10 mismatched donor were eligible. Reduced-intensity conditioning (RIC) regimen consisted of fludarabine 30mg/m²/day (d) from d-6 to d-2, busulfan 3,4 mg/kg/d from d-4 to d-3 and ATG (Thymoglobuline, Sanofi, Lyon, France) 2,5 mg/Kg/d, d-2 and d-1 (FB2A2) or the same but with clofarabine 30mg/m²/d in replacement of fludarabine with 1 or 2 d of ATG (CloB2A2/CloB2A1). Reduced-toxicity myeloablative conditioning regimens (RT-MAC) consisted of the same as FB2A2 but with 3 or 4 d of busulfan instead of 2 (FB3A2/FB4A2). All grafts were administered freshly on the day of the collection while patients (pts) had already received ATG. GVHD prophylaxis was ciclosporine alone for pts with a sibling donor while pts grafted with a matched (MUD) or a 9/10 mismatch (mmUD) unrelated donor received ciclosporine+MMF. We exhaustively looked at pts for whom a blood differential was available at the time of ATG administration in order to study the impact on OS, DFS and GRFS (no grade 3-4 acute GVHD, no moderate/severe chronic GVHD and no relapse) of a profound lymphopenia vs not.
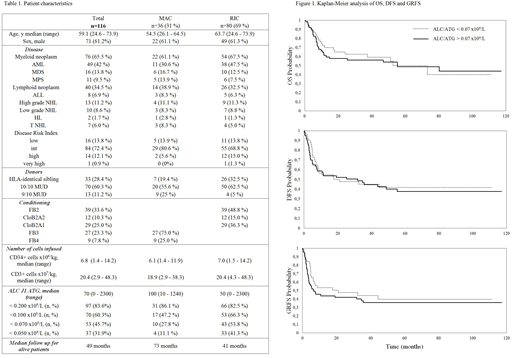
Results: Of 395 eligible pts, 116 (median follow-up for alive pts: 49 months) were documented with a differential at time of ATG administration, confirming that this analysis is not a routine practice in our department and probably in many centers. RIC was administered in 80 (69%) of the pts including 39 FB2A2, 12 CLOB2A2 and 29 CLOB2A1. RT-MAC was administered in 36 (31%) pts, including 27 FB3A2 and 9 FB4A2. Seventy-six pts had a myeloid disease while 40 had a lymphoid disease. Donor types were siblings (n=33), MUD (n=70) or 9/10 mmUD (n=13). For the entire cohort, 4y OS, DFS and GRFS were 56.2% (47-66), 40.9% (32-51) and 34.5% (26-45), respectively. No difference in survivals was observed between lymphoid vs myeloid pts, pts transplanted with sibling vs other donors, pts receiving a RIC vs a MAC or a CloB2 vs a FB2 RIC regimen.
Median ALC at time of start of conditioning was .915x109/L (range: .010-15.780). No difference in terms of survivals was observed when considering pts under this threshold vs others.
ROC curve analysis failed to identify a cut-off allowing to predict better survivals according to ALC at the time of ATG administration (ALC/ATG). Median ALC/ATG was .070x109/L (range: 0-2.300). No difference in terms of survivals was observed when considering pts under this threshold vs others. The same was true when considering .100x109/L as ALC/ATG cut-off. Regarding MAC, the median ALC/ATG was .100x109/L with no difference in survivals between pts under or above this value. The same was true for RIC with ALC/ATG cut-offs < median (.055x109/L) or <.100 x109/L. Interestingly, considering pts with ALC/ATG <.100 x109/L within the RIC setting, survivals were similar between those who received 1d (n=25) vs 2d (n=28) of ATG. This analysis was not performed for pts with ALC/ATG >.100 x109/L as only 4 of them received 1d of ATG vs 23 2d.
The dose of CD34+ and CD3+ T cells infused had no impact also on survivals.
Conclusion: This study demonstrates that profound lymphopenia at the time of ATG administration as part of a RIC as well as a RT-MAC Purine analogue/Busulfan/ATG based conditioning regimen has no impact on outcomes. Moreover, a reduced dose of ATG in RIC pts with profound lymphopenia at the time of ATG administration did not translate into better survivals. Other unknown factors rather than recipient lymphopenia remain to be discovered to optimize individualized dosing of ATG.
Peterlin:AbbVie Inc: Consultancy; Astellas: Consultancy; Jazz Pharma: Consultancy; Daiichi-Sankyo: Consultancy. Moreau:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Le Gouill:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Roche-Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Chevallier:Daiichi Sankyo: Honoraria; Incyte: Consultancy, Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.