Background
Persons with multiple sclerosis (MS) are sometimes treated with high-dose immune suppressive or cytotoxic drugs and an autotransplant. Use of autotransplants may be substantially more common as many cases are not reported. Therapy-related mortality (TRM) has decreased to <2% because of less intensive pretransplant regimens and better subject selection. Trials have reported a greater proportion of subjects with no evidence of active disease in subjects receiving high-dose therapy and an autotransplant compared with controls. Recent autotransplants use less intensive pretransplant therapy with seemingly similar efficacy and fewer adverse events. Whether the autograft is needed for bone marrow recovery and/or efficacy is uncertain but unlikely to be tested in randomized trials. We previously reported autotransplants for several haematologic neoplasms can be done in an outpatient setting using refrigerated blood cells. We now report data from 739 subjects with MS receiving autotransplants in an outpatient setting using refrigerated blood cells.
Methods
After June 2015, 739 consecutive patients with MS were autografted in a single center using non-frozen peripheral blood stem cells, on an outpatient basis and conditioning with cyclophosphamide and rituximab. The protocol was registered in ClinicalTrials.gov identifier NCT02674217. Eligibility criteria included all the following: Karnofsky performance score >70%, extended Disability Status Scale (EDSS ≤8 in the 2 w pretransplant), CNS magnetic resonance image (MRI) ≤3 mo pretransplant, no prior bone marrow toxic drugs, normal heart, liver, lung and kidney function, ≥6 mo since exposure to immune suppressive drugs.
Results
495 females and 244 males were included; median age was 47 years. 310 patients presented with relapsing remitting MS (RRMS), 273 with secondary progressive (SPMS) and 156 with primary progressive (PPMS). All procedures were started on an outpatient basis and only 31 persons needed to be admitted to the hospital during the procedure. In order to obtain at least 1x106/Kg viable CD34 cells, one to three apheresis were performed (median 1). Total number of viable CD34+ cells infused ranged between 1 and 37.83 x106 / Kg (median 5.62). Patients recovered above 0.5 x109/L absolute granulocytes on day 8 (median, range 2-13), whereas platelet recovery above 20 x109/L on day 4 (median, range 0-10). Seven individuals required red blood cells and eight needed platelet transfusions.
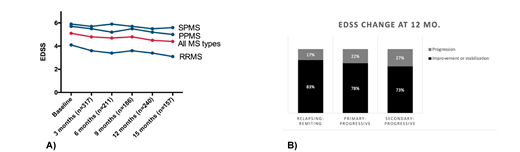
There was one transplant related death and the 30-month overall survival of the patients is 99.9%. Patients with RRMS or PPMS had a significant drop in the EDSS before and 15-mo after the transplant, whereas patients with SPMS remained stable (A). The response rate (either drop or stabilization of the EDSS score) at 12 months was 78% for RRMS, 81% for PPMS and 73% for SPMS (B), whereas the relapse-free survival was 84% for all patients (92% for PPMS, 83% for RRMS and 81% for SPMS).
Conclusions
Changes in the EDSS score consonant with neurological improvement were observed in persons with all types of MS after HSCT employing the "Mexican method".
Gomez-Almaguer:Amgen: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Teva: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.