Purpose: Dual expression of MYC and BCL2 proteins (Double Expressor Lymphoma-[DEL]) and MYC, BCL2 and /or BCL6 translocations (Double Hit Lymphoma-[DHL]) as well as the cell-of-origin (COO) are important prognostic factors in patients (pts) with diffuse large B-cell lymphoma (DLBCL) who are treated with standard chemo-immunotherapy. Data are limited regarding the prognostic impact and interdependence of these biomarkers on outcomes in pts with relapsed DLBCL treated with autologous stem cell transplantation (ASCT).
Methods: Data from Pts with relapsed DLBCL who underwent ASCT at our center and in whom archived tumor material was available were analyzed. Cutoff values of 40% for MYC and 70% for BCL2 were established by immunohistochemistry (IHC). FISH cases for MYC and BCL2 were considered for evaluation if at least 200 tumor cell nuclei per core displayed reliable signals in the sections. COO classification was achieved by IHC methods according to both the Visco-Young and Choi algorithms. The majority of pts (81%) underwent chemo-mobilization of stem cells with rituximab for in-vivo purging; rituximab was also given on days+1 and +8 with BEAM conditioning (J Clin Oncol 2005; 23:2240-7; Clin Cancer Res 2018; 24:2304-11). The study was IRB-approved at our center.
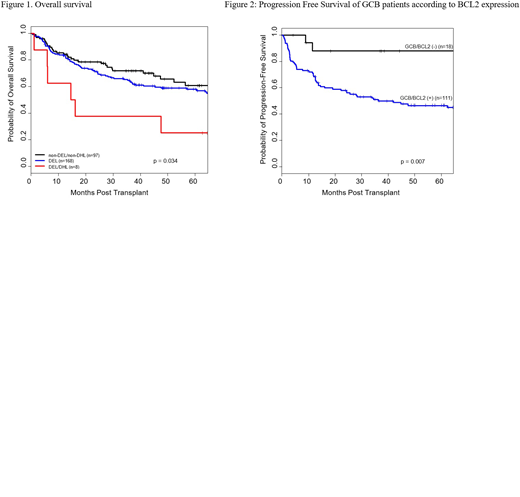
Results: 303 pts were evaluated; 169 (56%) met the criteria for DEL and 3 (1%) for DHL; 8 (3%) met criteria for both (DEL/DHL) and 97 (32%) for neither (non-DEL/non-DHL). Because of small size, the 3 pts with only DHL were excluded from this analysis. In addition, 8 pts (3%) had atypical DHL and their outcomes were analyzed separately. GCB classification was successful in 269 pts, and 119 pts (44%) were of the GCB subtype. Median age of the whole group was 60 years (range, 18-80); the male gender predominated (65%). The median number of prior lines of therapies was 2. At ASCT, 90% of pts had chemo-sensitive disease (64% CR, 26% PR), and 6% had small volume SD; IPI was ≥ 2 in 17% of pts and PET was positive in 26%. There were no statistically significant differences in pt characteristics between the subgroups of non-DEL/non-DHL, DEL, or DEL/DHL, with the exception of GCB distribution: 46% and 41% of non-DEL/non-DHL and DEL, respectively, were classified to be of the GCB subtype, whereas all 8 DEL/DHL were classified as non-GCB (P=0.002). With a median follow-up time among survivors of 50 months (range, 4-217 months), the 4-year overall survival (OS) rates of non-DEL/non-DHL, DEL and DEL/DHL subgroups were 65%, 59%, and 25%, respectively. There was no significant difference in OS between non-DEL/non-DHL and DEL subgroups (P=0.39), however a significant difference in OS was observed between the two subgroups compared to the DEL/DHL pts (P=0.034; Figure 1). Progression-free-survival (PFS) rates were higher for the non-DEL/non-DHL (4-year rate: 51%) and DEL (4-year rate: 49%) compared to DEL/DHL (4-year rate: 25%) subgroups, though not statistically different (P=0.22). A higher risk of non-relapse mortality was observed in the DEL/DHL group compared to the other 2 groups (hazard ratio [HR]=3.8, P=0.017). The 4-year OS and PFS rates for the atypical DHL were 67% and 33%, respectively. We also evaluated the interaction of COO with BCL-2 protein expression; we found that pts who had BCL2 (+) expression, had worse OS (HR=1.82; P=0.049) and a trend for worse PFS (HR=1.58; P=0.08) compared to BCL2 (-) pts. This interaction was more prominent however in GCB pts. The 4 year OS rates of GCB/BCL2(-) and GCB/BCL2(+) were 87% and 56%, respectively (P=0.030). The 4-year PFS rates were 88% and 47%, respectively (P=0.007) (Figure 2). The OS and PFS rates within non-GCB subgroups were similar regardless of BCL2 expression.
Conclusions: Our study shows that pt subgroups who have both DEL/DHL DLBCL have inferior survival. Interestingly, we also found that pts who have DEL and non-DEL/non-DHL have similar outcomes after ASCT. BCL2 expression is an important prognostic factor in GCB lymphoma. Investigational studies combining targeted therapies in this setting are warranted.
Jabbour:Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding. Molldrem:M. D. Anderson & Astellas Pharma: Other: Royalties. Bashir:Imbrium: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Spectrum: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; StemLine: Research Funding; Acrotech: Research Funding; Celgene: Research Funding. Kebriaei:Amgen: Research Funding; Pfizer: Honoraria; Jazz: Consultancy; Kite: Honoraria. Popat:Bayer: Research Funding; Incyte: Research Funding; Jazz: Consultancy. Westin:Novartis: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board, Research Funding; MorphoSys: Other: Advisory Board; Curis: Other: Advisory Board, Research Funding; Genentech: Other: Advisory Board, Research Funding; Unum: Research Funding; Kite: Other: Advisory Board, Research Funding; Juno: Other: Advisory Board; 47 Inc: Research Funding. Qazilbash:Bioclinical: Consultancy; Amgen: Consultancy, Other: Advisory Board; Autolus: Consultancy; Genzyme: Other: Speaker. Champlin:Johnson and Johnson: Consultancy; Actinium: Consultancy; Sanofi-Genzyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.