Introduction: Multiple myeloma (MM) remains incurable when treated with novel myeloma therapies in combination with high dose chemotherapy and autologous stem cell transplantation (ASCT). In contrast to the accelerated development of effective new drugs for induction, maintenance, and relapse, single-agent melphalan remains the standard for conditioning before ASCT. Is this phase II trial, we prospectively evaluated a conditioning regimen consisting of high-dose intravenous busulfan and melphalan followed by bortezomib (BUMELVEL), an agent shown to be potentially synergistic in combination with alkylating agents. We report our 7 year long term follow up with direct comparison to a contemporaneous CIBMTR cohort with the primary aim of both long term progression-free (PFS) and overall survival (OS).
Methods: This phase I/II study was conducted between July 2009 and May 2012 at Loyola University Medical Center. MM patients with any disease response following induction were enrolled at time of stem cell transplantation. 43 patients received BUMELVEL conditioning followed by ASCT on the phase II portion of this study. Busfulan was administered intravenously (i.v.) daily for 4 days with the first 2 days (days −6 and −5) at fixed dose of 130 mg/m2 over 3 hours and the subsequent 2 doses (days −4 and −3) adjusted to achieve a target AUC total of 20,000 μM·min determined by pharmacokinetic (PK) analysis. Melphalan at 140 mg/m2 and bortezomib at 1.6 mg/m2 were administered i.v. on days −2 and −1, respectively. Maintenance therapy was not planned. Patients received prophylaxis for oral mucositis with palifermin. Data from this phase II clinical trial were compared against a matched control cohort of contemporaneous North American MM patients from the CIBMTR database (n = 162) receiving conditioning with single agent iv melphalan at a dose of 200mg/m2. The primary objectives of this study were median PFS and toxicities.
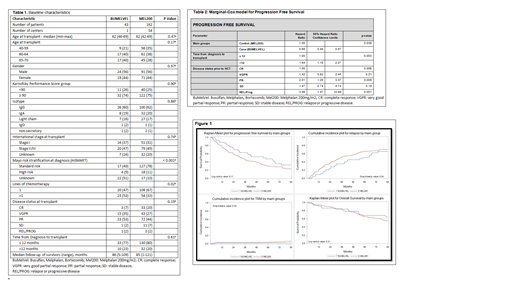
Results: Baseline patient characteristics are described in Table 1. More patients in the BUMELVEL arm received greater than one line of therapy prior to transplant (53 v 33%). There were more standard risk patients per Mayo Stratification (Kumar et al, Mayo Clin Proc 2009) in the MEL200 group (78%) v the BUMELVEL group (40%). There was no difference in response rates at day 100 (p=0.48) but a trend toward improved response at 1 year in the BUMELVEL arm with 77% achieving a VGPR or better versus 60% in the MEL200 group (p-0.09). No patients in the BUMELVEL group received planned maintenance therapy post-transplant whereas 112 (69.6%) in the MEL200 control arm received planned post-transplant maintenance. Non-relapse mortality was 2% (range 0-9%) in the BUMELVEL group and 10 (6%, range 3-11) in the MEL200 but this was not statistically significant in both univariate and multivariate modeling (p=0.25). There were no episodes of sinusoidal obstructive syndrome in the BUMELVEL group and 37% of patients experienced grade 3 mucositis with no grade 4 mucositis. After a median of more than seven years of follow up, the five year PFS was 47% (95% CI; 32-62) in the BUMELVEL group versus 30% (95% CI; 23-37) in the MEL200 group (p=0.05) (Figure 1). Notably the 7-year PFS in the BUMELVEL group was 32% (range 18-48). In multivariate analysis for PFS, BUMELVEL conditioning (HR 0.65; 95% CI 0.44-0.97)(p=0.036), time from diagnosis to transplant of greater than versus less than 12 months (HR 1.64; 95% CI 1.18 - 2.27), and disease status at transplant CR versus other (p=0.006) were all predictive of PFS (table 2). OS was not different between the two groups with a 7 year survival of 64% (95% CI; 48-79) in the BUMELVEL group versus 55% (95% CI; 46-64%) in the MEL200 group (Figure 1) which was confirmed on multivariate analysis (p=0.33).
Discussion: Similar to recent reports in the literature comparing busulfan and melphalan to melphalan alone (Bashir et al, Lancet Hem 2019), we show that although depth of response was similar between the BUMELVEL group and the historical MEL200 comparator, the BUMELVEL group experienced an improved PFS. Importantly, we report no cases of SOS and a low non-relapse mortality of only 2% (v 6% in the historical control) reinforcing that the preparatory regimen in myeloma can be intensified safely with improved duration of unmaintained PFS. These long term results confirm a sustained benefit of novel combination conditioning in MM and the need for novel transplant preparative regimens.
D'Souza:EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio: Research Funding; Prothena: Consultancy; Pfizer, Imbrium, Akcea: Membership on an entity's Board of Directors or advisory committees. Hari:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Amgen: Research Funding; Spectrum: Consultancy, Research Funding; Sanofi: Honoraria, Research Funding; Cell Vault: Equity Ownership; AbbVie: Consultancy, Honoraria. Stiff:Unum: Research Funding; Gamida-Cell: Research Funding; Incyte: Research Funding; Cellectar: Research Funding; Amgen: Research Funding; Gilead/Kite Pharma: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.