Introduction:
Histiocytic and dendritic neoplasms are a diverse group of uncommon hematologic tumors arising from monocytic or dendritic cell lineage. While genomic profiles for the more common entities Langerhans cell histiocytosis and Erdheim-Chester disease are established, less common neoplasms in this broad category are poorly characterized. In the current study, we assessed the genomic landscape of these often aggressive histiocytic and dendritic cell neoplasms to identify distinct mutational signatures for each subtype in the current WHO classification system.
Methods:
104 histiocytic and dendritic cell neoplasmswere tested during routine clinical care by hybridization capture of 406 cancer-related genes to detect base substitutions, small indels, amplifications (amp), deletions, and rearrangements. Tumor mutational burden (TMB, mutations/Mb) was determined on ~1.1 Mbp of sequenced DNA. Review of pathology reports, histopathology, and patient clinical data was performed. Cases included 48 follicular dendritic cell sarcoma (FDCS), 37 histiocytic sarcoma (HS), 8 interdigitating dendritic cell sarcoma (IDCS), 5 Langerhans cell sarcoma (LCS), 4 indeterminate cell histiocytosis (ICH), 1 fibroblastic reticular cell tumor (FRCT), and 1 inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcoma (FDC/FRCS).
Results:
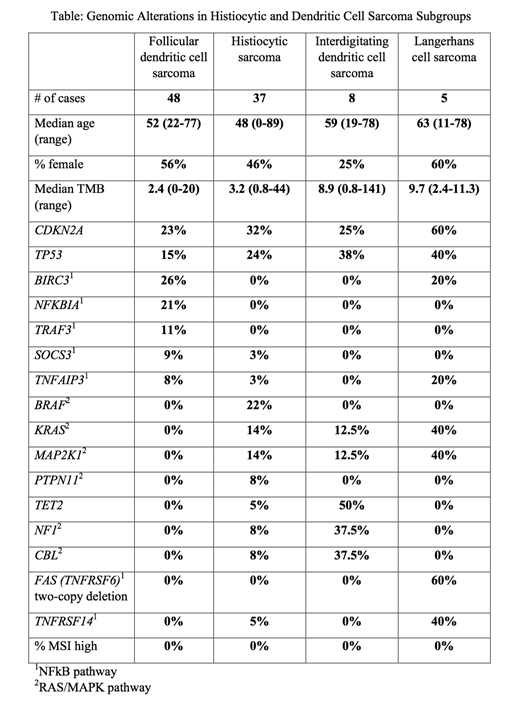
Pathogenic or likely-pathogenic genomic alterations (GAs) in the four most common sarcoma subgroups are summarized in the Table. CDKN2A and TP53 pathogenic mutations were the most frequent GAs observed in the cohort, present in 27% and 20% of the cases, respectively. Compared to the rest of the cohort, FDCS showed significantly more cases harboring a pathogenic GA in genes involved with NFkB pathway regulation (58% vs. 18%, p<0.0001). Other relevant alterations in FDCS included NTRK1 (2 cases; 1 NTRK1-PDIA3 fusion and 1 amp), NTRK3 (1 case), FGFR3 (1 amp), STAT3 (3 cases) and PTEN (3 cases). Compared to the rest of the cohort, HS showed significantly more cases with pathogenic GAs in the MAPK pathway (59% vs. 19%, p<0.0001), including 3 cases with BRAF fusions. Other relevant alterations in HS included PTEN (3 cases), PDGFRA (2 amp), MET (2 amp), 2 IGH-BCL2 fusions, and 1 IGH-BCL6 fusion. IDCS cases showed pathogenic MAPK GAs in 88% (7/8), and TET2 mutations were identified in 50% (4/8). A single NTRK1-TPR fusion was identified. LCS cases demonstrated GAs in both MAPK and NFkB pathways (4/5 and 3/5 cases, respectively). 2/4 cases of ICH demonstrated NCOA2-ETV3 fusions; TET2 mutations were present in the remaining two cases. The FRCT case contained an NRAS GA, and the FDC/FRCS cases had EBV (HHV-4) sequence reads.
Conclusions:
Our findings provide a comprehensive view of the shared and distinct genomic features among these rare histiocytic and dendritic cell neoplasms. The frequent inactivation of CDKN2A, which normally encodes an endogenous CDK inhibitor, suggests possible effectiveness of pharmacologic CDK4/6 inhibitors in treatment of this subset of cases. There are significant differences in the molecular profiles of different diseases: 58% of FDCS cases contain at least one pathogenic alteration in the NFkB pathway, while 59% and 88% of HS and IDCS cases contain potentially-actionable MAPK pathway mutations, respectively. LCS cases show frequent mutations in both pathways. Potentially-actionable molecular alterations are identified in 63/104 (61%) histiocytic and dendritic tumors in our cohort. These results illustrate the importance of performing comprehensive genomic profiling to define treatment strategies, including clinical trial "molecular eligibility" and more fully inform personalized therapeutic options.
Hasserjian:Jazz Pharmaceuticals: Consultancy; Promedior, Inc.: Consultancy. Montesion:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Sokol:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Pavlick:F. Hoffman La Roche, Ltd.: Equity Ownership; Foundation Medicine, Inc.: Employment. Shah:F. Hoffman La Roche, Ltd.: Equity Ownership; Foundation Medicine, Inc.: Employment. Danziger:F. Hoffman La Roche, Ltd.: Equity Ownership; Foundation Medicine, Inc.: Employment. Killian:F. Hoffman La Roche, Ltd.: Equity Ownership; Foundation Medicine, Inc.: Employment. Severson:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Duncan:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Elvin:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Miller:F. Hoffman La Roche, Ltd.: Equity Ownership; Foundation Medicine, Inc.: Employment. Ross:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Vergilio:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership. Williams:Foundation Medicine, Inc.: Employment; F. Hoffman La Roche, Ltd.: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.