Background. Comorbidities have a well-established prognostic value in the majority of hematologic neoplasms. However, the impact of comorbidities on adult patients with acute lymphoblastic leukemia (ALL) has been poorly investigated to date.
Aims. In this retrospective study of a monocentric cohort of 112 adults with Philadelphia-negative (Ph-neg) ALL consecutively diagnosed between 1990 and 2018 we aimed to assess the association between comorbidities at diagnosis and remission rate, the frequency and severity of adverse events (AE) during frontline treatment, and long-term outcome.
Patients and methods. Patients were included, regardless of age, if treated within or according to clinical protocols aimed at curing. Patients initially treated with low-intensity/palliative care were not considered. Ph-positive patients were excluded, as their treatment modalities markedly changed over years due to the availability of tyrosine kinase inhibitors. Patients were defined as high risk if one or more of the following features were present: WBC >30x109/L for B-cell precursor (BCP) or >100x109/L for T-cell precursor (TCP), pro-B, early or mature T ALL phenotype, high risk cytogenetics (according to the ESMO 2016 guidelines), or central nervous system involvement. Comorbidities recorded at diagnosis were classified according to the Charlson Comorbidity Index (CCI). Classification and grading of AE was performed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Responses were defined according to the ESMO 2016 guidelines. Minimal residual disease (MRD) was assessed by 6- or 8-colour flow cytometry with a 10-4 sensitivity. Variables tested for association with complete remission (CR) and MRD-negative status were: age group (stratified into quartiles), gender, phenotype (BCP or TCP), WBC, karyotype, risk class, and CCI score. Event-free survival (EFS) was calculated from the first CR to relapse or death. Overall survival (OS) was calculated from diagnosis until death. EFS and OS were estimated using the Kaplan-Meier method.
Results. Median age at diagnosis was 43 years (range 14-75). BCP and TCP were 78% and 22%, respectively. Overall, 51 patients (46%) were considered as standard risk, 46 (41%) as high risk, and 15 (13%) were unclassifiable due to the lack of cytogenetics. CCI score distribution was as follows: 0 (n=66; 59%), 1 (n=18; 16%), 2 (n=9; 8%), and ≥3 (n=19; 17%).
CR after induction therapy was obtained by 95/112 patients (85%). CCI was the only variable significantly associated to CR achievement (0-1 vs ≥2: 91% vs 68%; p=0.012). MRD status at the end of induction was evaluable in 64 out of 95 patients in CR and was negative in 28 (44%). No pretreatment variable predicted for the achievement of MRD complete response. Eight patients (7%) died before the first response evaluation; early death rate was significantly higher in patients with CCI ≥2 than in patients with CCI 0-1 (21% vs 2%; p=0.003). Conversely no differences in AE frequency and severity were observed according to CCI (p=0.847) or age group (p=0.881).
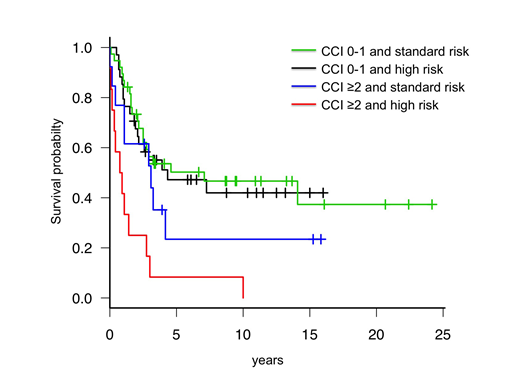
After a median follow-up of 35 months, 46 patients (41%) were alive. OS was significantly longer in patients with CCI 0-1 as compared to CCI ≥2 (median 87 months, 95%CI: 31 to not reached vs 13 months, 95%CI: 5-36; p<0.001). Notably, pre-treatment risk stratification lost prognostic value in predicting OS and EFS in CCI 0-1 patients, while in CCI ≥2 patients retained its significance (Figure). Overall, 58 patients (52%) were primary refractory or relapsed. Relapse rate was similar according to age groups, phenotype, risk class, and CCI score. CR rate after salvage treatment (CR2) was higher in younger patients (≤43 vs >43 years: 81% vs 44%; p=0.014) and tended to be higher in patients treated with blinatumomab or inotuzumab rather than with standard chemotherapy (82% vs 55%, p=0.17). While there were no differences in CR2 according to CCI group, median survival after first relapse was 12.1 months (95%CI: 7.83-15.24) for CCI 0-1 vs 6.92 months (95%CI: 2.49-12.59) for CCI≥2(p=0.021).
Conclusions. Comorbidities have a significant prognostic impact in intensively-treated adults with Ph-neg ALL. Patients with low comorbidities (CCI 0-1) had superior CR rates and less incidence of toxic death after induction, and pre-treatment high risk features did not have a detrimental impact in this group.
Krampera:Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Bonifacio:Incyte: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Research Funding; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.