Introduction: Tandem autologous transplant (auto- auto) has been studied as a method to increase remission rates and reduce relapse in the upfront therapy of MM. The use of autologous followed by reduced intensity conditioning allogenic transplantation (auto-allo) offers the potential of long-term graft-versus-myeloma (GVM) effect, but with the risk of graft versus host disease and potentially higher non-relapse mortality (NRM). Trials comparing these two strategies relied on availability of HLA-matched sibling donors for arm allocation (biological "randomization") and have yielded conflicting results, in part due to trial size or limited follow up. A pooled analysis of multiple trials with extended follow up provides the best opportunity to compare these two transplant strategies.
Methods: We obtained individual patient data from participants of 4 trials comparing auto-auto vs. auto-allo after brief induction therapy, namely BMT CTN 0102 (N=709), NMAM2000 (N=357), PETHEMA/GEM2000 (N=110), and the Torino consortium trial (N=162). In all 4 trials arm allocation was by biological "randomization". Patients were designated high risk if beta-2 microglobulin ≥ 4.0 mg/L at diagnosis or presence of deletion of chromosome 13 by metaphase karyotyping. Time to event outcomes were analyzed by intention to treat, from the time of first autologous transplant. Main outcomes analyzed were overall survival (OS) and progression free survival (PFS). Secondary outcomes analyzed were NRM and risk of relapse, treated as competing risks, and post relapse survival.
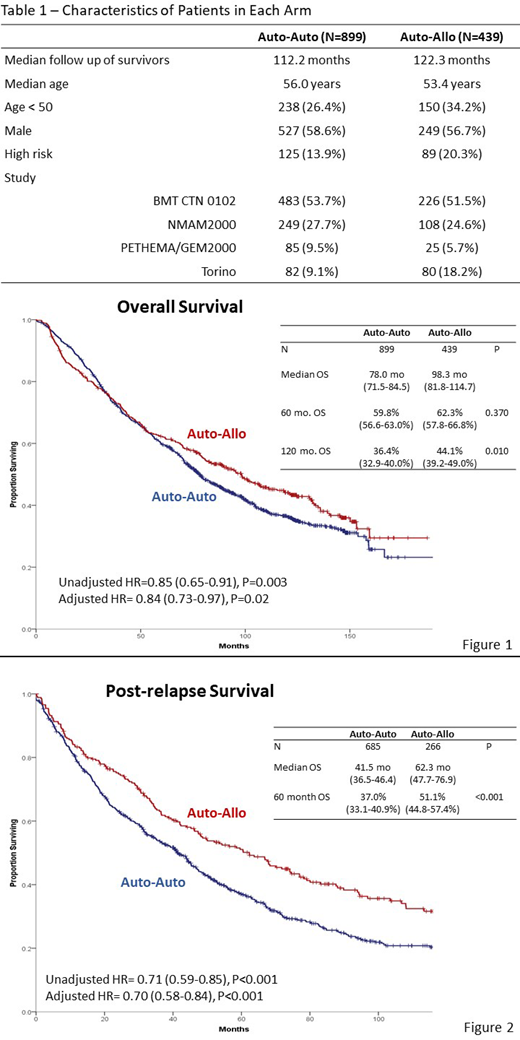
Results: There were 1,338 patients included in the analysis, 899 in auto-auto and 439 in auto-allo. Median follow up of survivors is 118.5 months. Characteristics of the two arms are displayed in Table 1. Median OS was 78.0 months in auto-auto and 98.3 months in auto-allo (HR= 0.85, P=0.003, Figure 1). OS was 59.8 % vs. 62.3% at 5-years (P=0.37) and 36.4% vs. 44.1% at 10 years (P=0.01) for auto-auto and auto-allo respectively. PFS was also improved in auto-allo (HR= 0.84, P=0.004) with 5-year PFS of 23.4 vs. 30.1% (P=0.01) and 10-year PFS of 14.4% vs. 18.7% (P=0.06). For the 214 high risk patients (125 auto-auto, 89 auto-allo) there was superior 5-year and 10-year PFS with auto-allo, but no difference in OS. Risk of NRM was higher in auto-allo (10 year 8.3% vs. 19.7%, P<0.001), while risk of disease progression was higher in auto-auto (10 year 77.2% vs. 61.6%, P<0.001). There were 685 progressions in auto-auto and 266 in auto-allo. Median post relapse overall survival was 41.5 months in auto-auto and 62.3 months in auto-allo (HR= 0.71, P<0.001, Figure 2). Five years post relapse, 37.0% of patients were alive in auto-auto vs. 51.1% in auto-allo (P<0.001).
Conclusion: Long-term follow up using a large pooled dataset of 4 trials indicates durable, long-term disease control with an auto-allo strategy. Despite higher NRM, there was a reduction in the risk of relapse and superior post relapse survival in auto-allo. This supports the hypothesis of a durable GVM effect enhancing myeloma control with subsequent therapies.
Costa:Janssen: Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy; Karyopharm: Consultancy; Fujimoto Pharmaceutical Corporation Japan: Other: Advisor; Sanofi: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding. Pasquini:Pfizer: Consultancy; Medigene: Consultancy; Amgen: Consultancy; Novartis: Research Funding; Kite Pharmaceuticals: Research Funding; BMS: Research Funding. Bladé:Janssen, Celgene, Amgen, Takeda: Membership on an entity's Board of Directors or advisory committees; Irctures: Honoraria. Schonland:Sanofi: Research Funding; Takeda: Honoraria, Research Funding; Prothena: Honoraria; Medac: Other: Travel grant; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hari:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Amgen: Research Funding; Spectrum: Consultancy, Research Funding; Sanofi: Honoraria, Research Funding; Cell Vault: Equity Ownership; AbbVie: Consultancy, Honoraria. Giralt:Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Kite: Consultancy; Johnson & Johnson: Consultancy, Research Funding; Actinium: Consultancy, Research Funding; Miltenyi: Research Funding; Spectrum Pharmaceuticals: Consultancy; Amgen: Consultancy, Research Funding. Patriarca:Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Stadtmauer:Abbvie: Research Funding; Janssen: Consultancy; Amgen: Consultancy; Novartis: Consultancy, Research Funding; Tmunity: Research Funding; Celgene: Consultancy; Takeda: Consultancy. Krishnan:Celgene, Janssen, Sanofi, BMS: Consultancy; Sutro BioPharma, zPredicta: Consultancy; Amgen, Takeda: Speakers Bureau; Celgene, Z Predicta: Other: Stock Ownership; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.