Introduction: Mutations of the NPM1 gene (NPM1mut) are considered a favorable prognostic marker in acute myeloid leukemia (AML). Data from a prospective randomized trial have shown that in young AML patients with NPM1mut upfront allogeneic stem cell transplantation (allo-SCT) performed in first complete remission (CR1) may lead to a significantly improved overall survival (OS) (Dohner K, Blood 2005). However, due to considerable allo-SCT-associated morbidity and mortality and a relatively favorable outcome of these patients outside the transplant setting, many physicians are reluctant to refer NPM1mut AML patients to allo-SCT in CR1. Recently, quantitative NPM1 testing has been adopted in patient selection for allo-SCT; yet, evidence to support such practice is scant. The current single-center retrospective study compared the outcome of fit NPM1mut AML patients before and after the introduction of quantitative PCR monitoring to the routine practice at the Rambam Leukemia Unit.
Methods: This retrospective cohort analysis included NPM1mut AML patients who were considered fit for intensive chemotherapy. FLT3 positivity was not an exclusion criterion. The cohort incorporated patients treated between 2011-2014 whose minimal residual disease (MRD) status was not evaluated (n=31) and patients treated between 2015-2019 who underwent MRD assessment as part of their routine follow-up (n= 38). All patients (n=69) received intensive chemotherapy (the "3+7" regimen). In the former period, our practice was to recommend allo-SCT for any NPM1mut fit patient with an available donor. Starting from 2015, bone marrow samples of all patients have been monitored for MRD both post-induction and after each consolidation cycle, using quantitative PCR. Presently, only patients failing to achieve a 3-log reduction in the NPM1mut relative expression level (NPM1/ABL) after the first consolidation are recommended to proceed to allo-SCT during CR1. In case of molecular relapse identified in former MRDneg patients, the aim is to immediately perform allo-SCT in an attempt to avoid morphological relapse. Patient demographics, comorbidities, cytogentic and molecular risk factors were compared. Survival curves were calculated with and without censoring at time of SCT.
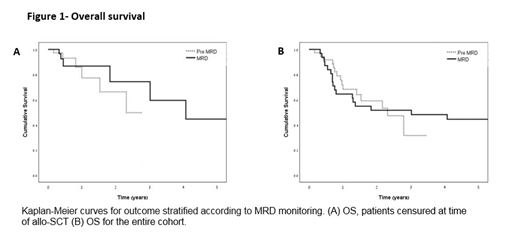
Results: Comparison of the pre-MRD monitoring cohort with the MRD-evaluated cohort revealed no difference in age (53.5 years and 56.2 years, respectively), comorbidities (ischemic heart disease, insulin-dependent diabetes mellitus, chronic kidney disease grade >2, chronic obstructive pulmonary disease and cirrhosis), high risk features such as FLT3 positive status, complex karyotype or P53 positive (38.8% and 39.5%, respectively) and extramedullary involvement (3.2% and 15.8%, respectively; P=0.12). More women were treated in the earlier period (64.5% and 39.5%, respectively; P=0.053). The use of intensified induction (daunorubicin 90mg/m2 or re-induction) was significantly more prevalent in the second cohort (50%, n=19) than in the first one (12.9%, n=4). Patients were generally prescribed the daunorubicin dose of ≥60mg/m2 or similar, apart from one patient in each group who received high-dose Ara-C as induction therapy. The use of MRD quantitative monitoring allowed reducing the portion of patients transplanted in CR1 from 61% (n=19/31) to 47.3% (n=18/38). Four patients who had not been transplanted in first molecular remission were successfully transplanted at time of molecular relapse without requiring salvage chemotherapy and prior to morphological relapse. To date, these four patients are alive and in remission at a follow-up of 1,8, 17 and 26 months from relapse. The 1- and 2-year OS was identical in the two cohorts (1 year: 77.3% vs. 86.7%; 2 years: 66.3% vs 74.4%, respectively) (Fig 1a). There was no significant change without censoring at time of SCT (Fig 1b).
Conclusions: The present analysis demonstrates that in clinical practice quantitative NPM1 monitoring may be safely used for precise selection of NPM1mut fit AML patients for allo-SCT. This approach may allow avoiding transplantation in patients who may have a prolonged remission outside the transplant setting, while reserving allo-SCT for the time when such patients experience molecular relapse. The use of MRD monitoring as decision making tool in the hematologist armamentarium should be evaluated in lager patient cohorts.
Author notes
Asterisk with author names denotes non-ASH members.