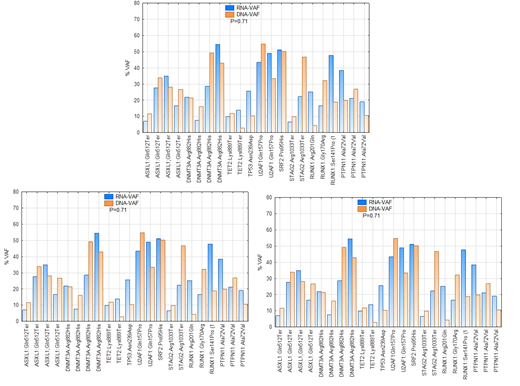
Introduction: Isocitrate dehydrogenase 1 and 2 (IDH1/2) are homodimeric enzymes that play an important role in cellular metabolism, epigenetic regulation, and DNA repair. Early studies suggested that mutations in IDH1/2 were loss of function mutations associated with a tumor suppressor function. However, biallelic mutations are extremely rare, and studies demonstrate that mutant IDH1/2 enzymes are responsible for NADPH-dependent reduction of αKG to the oncometabolite d-2-hydroxyglutarate (D2HG), suggesting an oncoprotein. Cellular RNA levels are tightly regulated by very complex cellular processes, and the regulation of mutant mRNA in cancer cells is rarely studied. We explored the effects of IDH1/2 mutations on mRNA levels in patients with Acute myeloid leukemia (AML). Using next generation sequencing (NGS) and variant allele frequency (VAF) of mutant RNA, we compared relative mutant mRNA or variant allele frequency (RNA-VAF) with variant allele frequency of mutant DNA (DNA-VAF) in the same samples from patients with AML. Methods: RNA and DNA were extracted from 48 bone marrow and peripheral blood samples from patients with confirmed AML, including 12 patients with IDH1 mutations, 2 with IDH2 mutation and 34 samples from AML without IDH1/2 mutations. Samples were collected pretherapy as well as while on therapy. We sequenced the DNA using 177 gene panel and the RNA using 1408 gene panel. The DNA sequencing is based on Single Primer Extension (SPE) library preparation with unique molecular identifier (UMI) (Qiagen, Germantown, MD). Average coverage of DNA sequencing was >1000X. The RNA sequencing is based on hybrid capture and the number of reads ranged from 5 to 10 million. Sequencing data of DNA is analyzed using the DRAGEN Platform. Sequence duplicates were removed before calculating VAF. The RNA sequencing data is analyzed using Illumina basespace. RNA VAF is calculated also after removing duplicates using Isaac variant caller. Only mutations detected by both DNA and RNA variant callers are compared. Results: A total of 176 mutations were detected using the DNA panel and 122 mutations using the RNA panel. Some mutations were called by RNA variant caller, but not by DNA variant caller and vice versa. All mutations detected in IDH1 and IDH2 were detected in both DNA and RNA. When the IDH1/2 mutations are considered (#14), the VAF in RNA (median: 41%, range: 13%-74%) was significant higher (P=0.006, Wilcoxon matched pairs test ) as compared with DNA (median:28%, range: 13%-74%). The VAF of the other 31 mutations that were detected in both DNA and RNA varied dependent on the gene. ASXL1, DNMT3A, RUNX1, PTPN11, SRSF2, STAG2 and U2AF1 mutations showed no significant difference between DNA and RNA in VAF (P=0.71). Although the number is small, mutations in NRAS and NPM1 showed significantly higher VAF in RNA as compared with with that of DNA (P=0.008). Conclusion: This data suggests that, in general, stability of mutant RNA varies between genes and between the mutations in the same gene. Mutant IDH1/2 RNA is significantly more stable in myeloid leukemic cells a compared with the wild-type mRNA. Most likely this reflects increased levels of mutant IDH1/2 as compared with wild-type IDH1/2, confirming that IDH1/2 is oncoprotein and may explain the efficacy of therapeutic inhibition of IDH1/2 in treating cancers. Furthermore this suggests that mRNA testing might be more sensitive in monitoring minimal residual disease in patients with IDH1/2 mutations.
Albitar:Genomic Testing Ccoperative: Employment, Equity Ownership. Konopleva:Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding. Loghavi:GLG Consultants: Consultancy; AlphaSights: Consultancy; MDACC: Employment. Takahashi:Symbio Pharmaceuticals: Consultancy. Kantarjian:Jazz Pharma: Research Funding; Pfizer: Honoraria, Research Funding; Ariad: Research Funding; Cyclacel: Research Funding; Novartis: Research Funding; Astex: Research Funding; Takeda: Honoraria; Agios: Honoraria, Research Funding; BMS: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding. DiNardo:medimmune: Honoraria; agios: Consultancy, Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; jazz: Honoraria; abbvie: Consultancy, Honoraria; celgene: Consultancy, Honoraria; daiichi sankyo: Honoraria; syros: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.