Background: The Janus kinase (JAK) inhibitor ruxolitinib (RUX) is approved for the treatment of disease-related splenomegaly and symptoms in patients with myelofibrosis (MF). Treatment with RUX has been shown to significantly reduce splenomegaly and provide marked improvements in MF-related symptoms and quality-of-life. A number of mutations have been identified with known or likely functional significance in patients with MF (Vannucchi AM, et al. Leukemia. 2013), which may therefore have the potential to affect treatment response. This exploratory analysis aimed to investigate the mutational status of patients in the REALISE trial and to assess the relationship between baseline mutational status and outcome.
Methods: REALISE was a multicenter, open label, single arm phase 2 study (NCT02966353). Eligible patients (N=51) had primary MF, post-essential thrombocythemia (ET) MF or post-polycythemia vera (PV) MF, with palpable (≥5 cm) spleen and hemoglobin level <10 g/dL. Patients started RUX at 10 mg bid with up titrations to 15 or 20 mg bid allowed after 12 weeks based on efficacy and platelet counts. The primary endpoint was achievement of ≥50% reduction in spleen length at Week 24. Secondary endpoints included transfusion requirements/dependence over time, adverse events, and patient-reported outcomes (PRO) (7-point MF score [MF-7], MF Symptom Assessment Form [MFSAF] version 2.0). Next generation sequencing (NGS) analysis using a 236 gene panel (Navigate BioPharma, Carlsbad, CA, USA) was performed on whole blood samples to identify genetic alterations.
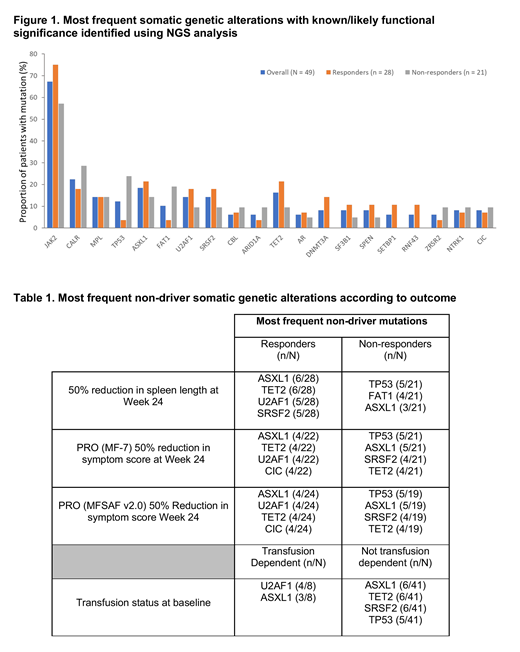
Results: NGS analysis data were available for 49/51 patients, median age was 67 years, 67.3% (33/49) had primary MF, 10.2% (5/49) had post-PV MF and 22.4% (11/49) had post-ET MF. DIPSS was available for 45 patients, 16.3% (8/49) were intermediate (Int)-1, 57.1% (28/49) were Int-2 and 18.4% (9/49) were high risk. The most frequent baseline mutations are shown in Figure 1. Classic driver mutations were found in JAK2 (n=33), CALR (n=11) and MPL (n=7), and did not affect response to RUX treatment. Two patients (4.1%) were triple negative for JAK2/CALR/MPL mutations, both responded to RUX treatment. The most commonly found non-driver mutations in patients with ≥50% reduction in spleen length at Week 24 (n=28) were TET2, ASXL1, U2AF1 and SRSF2; in non-responders, the most common non-driver mutations were TP53, FAT1 and ASXL1. The median number of mutations per patient was 2 (range 1-7); 35.7% (10/28) of patients with a response had ≥3 non-driver mutations vs 14.3% (3/21) of non-responders. Overall, no difference was seen in mutational distribution by change in spleen length at Week 24. In general, similar findings were seen for transfusion dependence status at baseline and improvements in symptom score with treatment (Table 1). However, there was a higher incidence of U2AF1 mutation in patients who were transfusion-dependent at baseline vs. non-transfusion dependent patients (4/8 [50%] vs 3/41 [7.3%], respectively). U2AF1 mutation is known to be associated with anemia and/or thrombocytopenia in myelodysplastic syndromes (Li B, et al. Genes Chromosomes Cancer. 2018). Mutations in TP53 were present in 6 patients. One patient showed a response to treatment at Week 24, and 5 were classified as non-responders. None of these 5 patients completed 24 weeks of treatment and 3 died during the study or safety follow-up period. Two progressed to acute myeloid leukemia prior to death. All patients were ≥60 years old, 4 were male and 4 were DIPSS Int-2 risk. Four patients had primary MF, 1 had post-ET MF and 1 had post-PV MF.
Conclusions: Though these data should be interpreted with caution due to the small patient numbers, patients in the REALISE study showed variation in the type and number of genetic alterations with known/likely functional significance in MF. Compared with published mutational data on MF patients treated with RUX (Spiegel, et al. Blood Advances. 2017; Pacilli et al. Blood Cancer Journal. 2018) 12.2% of patients had a TP53 mutation compared to 4% and 4.2% respectively. This molecular difference may reflect the anemic study population. Despite the higher TP53 mutational burden, and alternative dosing strategy, 57.1% (28/49) patients had a response to RUX at Week 24. Although there was no strong association between mutation patterns and response, patients with TP53 mutations tended to have a poor outcome overall.
Al-Ali:Celgene: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; CTI: Honoraria. Gisslinger:Novartis Pharma GmbH: Consultancy, Honoraria, Research Funding; Roche Austria GmbH: Consultancy; Myelopro GmbH: Consultancy; Celgene GmbH: Honoraria; Pharma Essentia: Other: Personal fees; Janssen-Cilag: Honoraria; AOP Orphan Pharmaceuticals: Consultancy, Honoraria, Research Funding. Passamonti:Janssen: Honoraria, Other: Advisory board , Speakers Bureau; Novartis: Honoraria, Other: Advisory board , Speakers Bureau; Celgene: Honoraria, Other: Advisory board , Speakers Bureau. Foltz:Novartis: Consultancy, Honoraria, Research Funding; Celgene: Consultancy; Amgen: Other: Spouse Employment; Incyte: Research Funding; Constellation Pharma: Research Funding. Ross:Celgene: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Vannucchi:Incyte: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Komatsu:Pharma Essentia: Research Funding, Speakers Bureau; Novartis K.K: Speakers Bureau; Wako Pure Chemical Industries, Ltd.: Research Funding; Takeda Pharmaceutical Company Limited: Research Funding, Speakers Bureau; Fuso Pharmaceutical Industries, Ltd.: Research Funding. Tiwari:Novartis: Employment. Zor:Novartis: Employment. Chaturvedi:Novartis Pharmaceuticals: Employment. Gilotti:Novartis Pharmaceuticals: Employment. Cervantes:Novartis: Honoraria, Speakers Bureau; Celgene: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.