Introduction:
Proteasome inhibitors (PI), immunomodulatory agents (IMiDs) and the CD38 monoclonal antibody daratumumab (dara) have transformed the management of MM, yet eventual refractoriness to these agents seems inevitable. Relapsed and refractory MM (RRMM) which becomes triple class refractory (TCR, i.e. refractory to a PI, an IMiD and Dara) uncommonly responds to further lines of therapy and survival is dismal. Selinexor is a selective inhibitor of nuclear export compound targeting exportin 1 (XPO1) which is overexpressed in MM cells and essential for their survival. In the STORM study, selinexor in combination with low-dose dexamethasone (Sd) demonstrated promising efficacy in TCR, penta-exposed (TCR-PE, i.e. exposed to lenalidomide, pomalidomide, bortezomib, carfilzomib and dara) MM. Establishing the natural history for outcomes in the TCR-PE population can help provide context to understand the outcomes observed with Sd in STORM. In the retrospective MAMMOTH study, we reported the outcomes of patients with RRMM after they became refractory to dara, including a subset of patients who were TCR. We further analyzed the MAMMOTH dataset to generate a cohort of patients similar to patients in STORM in order to compare conventional care vs. Sd.
Methods:
We included all patients in STORM who received Sd as the first line therapy after they achieved TCR-PE status (n=64). We extracted from the MAMMOTH dataset all patients who were not exposed to Sd in a subsequent line of therapy, became TCR-PE and who received subsequent MM-directed therapy (n=128). Overall response rate (ORR) was evaluated according to IMWG criteria. Overall survival (OS) was calculated from the time of initiation of next line of therapy after TCR-PE status until death or last follow-up. We compared OS in STORM vs. MAMMOTH utilizing cox-regression analysis with adjustment for covariables potentially influencing the outcome.
Results:
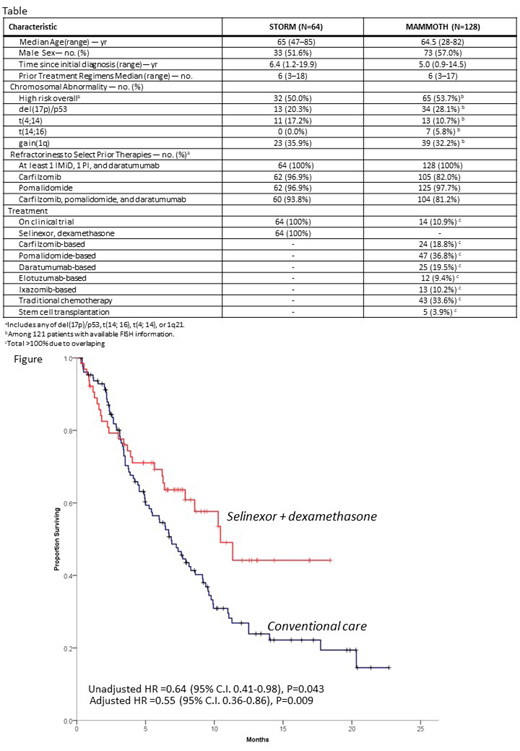
Baseline patient characteristics and prior therapies are per table. The two cohorts were similar in terms of age, number of prior lines of therapy and presence of high-risk cytogenetic abnormalities. STORM patients had longer time between MM diagnosis and post TCR-PE therapy with a higher proportion of refractoriness to carfilzomib. Patients in STORM had ORR to Sd of 32.8% vs 25.0% for patients receiving conventional care in MAMMOTH (P=0.078). In direct comparison, patients in STORM had better OS than patients in MAMMOTH (median 10.4 vs. 6.9 months) (P=0.043, figure). In multivariate analysis, STORM patients had lower risk of death in comparison with MAMMOTH patients (aHR=0.55, 95%C.I. 0.35-0.86, P=0.009). Refractoriness to carfilzomib (aHR=2.20, 95%C.I. 1.16-4.15, P=0.015) and high-risk cytogenetics (aHR-1.66, 95% C.I. 1.13-2.42, P=0.009) were also associated with inferior OS.
Conclusion:
Despite inherent limitations in comparison of trial enrollees vs. real world patients, this analysis suggests improved OS with Sd vs conventional care in patients with TCR-PE RRMM. Prognosis for these patients remains poor and underscores the need for therapeutic advancements.
Costa:Janssen: Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy; Karyopharm: Consultancy; Fujimoto Pharmaceutical Corporation Japan: Other: Advisor; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Hari:Kite: Consultancy, Honoraria; Amgen: Research Funding; Spectrum: Consultancy, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Consultancy, Honoraria; BMS: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Cell Vault: Equity Ownership; AbbVie: Consultancy, Honoraria. Kumar:Janssen: Consultancy, Research Funding; Takeda: Research Funding; Celgene: Consultancy, Research Funding. Tang:Karyopharm: Employment. Shah:Karyopharm Therapeutics Inc: Employment, Equity Ownership. Jagannath:BMS: Consultancy; Merck: Consultancy; Medicom: Speakers Bureau; Celgene: Consultancy; Novartis: Consultancy; Multiple Myeloma Research Foundation: Speakers Bureau. Chari:Oncoceutics: Research Funding; Novartis Pharmaceuticals: Research Funding; GlaxoSmithKline: Research Funding; Array Biopharma: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding. Shacham:Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Ma:Karyopharm: Employment, Equity Ownership. Siegel:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Biran:Amgen: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Bristol Meyers Squibb: Research Funding. Lonial:BMS: Consultancy; Genentech: Consultancy; GSK: Consultancy; Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Amgen: Consultancy; Celgene Corporation: Consultancy, Research Funding; Karyopharm: Consultancy. Richardson:Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding. Kauffman:Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Malek:Adaptive: Consultancy; Amgen: Speakers Bureau; Janssen: Speakers Bureau; Celgene: Consultancy; Takeda: Consultancy; Sanofi: Consultancy; Medpacto: Research Funding. Fiala:Incyte: Research Funding. Usmani:Amgen, Celgene, Janssen, Sanofi, Takeda: Speakers Bureau; Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, SkylineDX, Takeda: Other: Consultant/Advisor; Amgen Array Biopharma, Bristol-Myers Squibb, Celgene, Janssen, Merck, Pharmacyclics, Sanofi, Takeda: Other: Research Grant. Kang:Takeda Oncology: Consultancy; InCyte Corportation: Research Funding. Cornell:Takeda: Consultancy; KaryoPharm: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.