Background
Transplant non-eligible (TNE) myeloma patients are a very heterogeneous group that is not well-defined on the basis of age alone, but rather by the interplay of age, physical function, cognitive function and comorbidity better defined as 'frailty'. The International Myeloma Working Group (IMWG) has published a scoring system for myeloma patient frailty that predicts survival, adverse events and treatment tolerability using age, the Katz Activity of Daily Living (ADL), the Lawton Instrumental Activity of Daily Living (IADL), and the Charlson Comorbidity Index (CCI). It has been proposed to be useful in determining the feasibility of treatment regimens and appropriate dose reductions but has not been validated prospectively.
We hypothesize that by defining subgroups of patients based on the IMWG frailty score, and guiding up-front dose adjustments we can personalize therapy to improve treatment tolerability and therefore short-term outcomes, along with quality of life. In addition we plan to compare the use of single agent immunomodulatory (IMiD) based maintenance therapy with an IMiD and proteasome inhibitor maintenance doublet to try and improve long-term outcomes for patients.
Study Design and Methods
Myeloma XIV (NCT03720041) is a phase III, multi-center, randomized controlled trial to compare standard (reactive) and frailty-adjusted (adaptive) induction therapy delivery with the novel triplet ixazomib, lenalidomide and dexamethasone (IRd), and to compare maintenance lenalidomide (R) to lenalidomide plus ixazomib (IR) in patients with newly diagnosed multiple myeloma not suitable for a stem cell transplant.
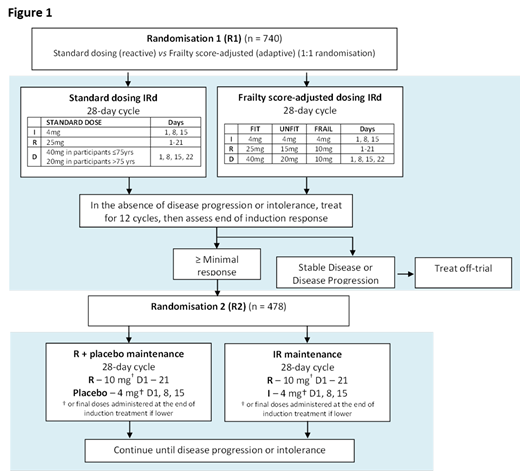
The trial outline is shown in Figure 1. All participants receive induction treatment with ixazomib, lenalidomide and dexamethasone and are randomized (R1) on a 1:1 basis at trial entry to the use of frailty score-adjusted up-front dose reductions vs. standard up-front dosing followed by toxicity dependent reactive dose modifications during therapy. Following 12 cycles of induction treatment participants alive and progression-free undergo a second randomization (R2) on a 1:1 basis to maintenance treatment with lenalidomide plus placebo versus lenalidomide plus ixazomib. Participants and their treating physicians are blinded to maintenance allocation.
The primary objectives of the study are to determine:
Early treatment cessation (within 60 days of randomization) for standard versus frailty-adjusted up-front dosing
Progression-free survival (PFS, from maintenance randomization) for lenalidomide + placebo (R) versus lenalidomide + ixazomib (IR)
The secondary objectives of the study include determining: progression-free survival (PFS) for standard versus frailty-adjusted up-front dosing reductions, overall survival (OS), overall response rate (ORR), treatment compliance and total amount of therapy delivered, toxicity & safety including the incidence of Second Primary Malignancies (SPMs), Quality of Life (QoL), cost-effectiveness of standard versus frailty-adjusted up-front dosing of IRd and cost-effectiveness of IR versus R. Exploratory analyses include the association of molecular subgroups with response, PFS and OS.
Seven hundred and forty participants will be enrolled into the trial at R1 to give 80% power to demonstrate a difference in early cessation and ensure that at least 478 participants remain and are randomized at R2 (based on attrition rates in our previous study Myeloma XI). At R2 478 patients will give us 80% power to detect an eight month difference in PFS between R and IR.
Cairns:Celgene, Amgen, Merck, Takeda: Other: Research Funding to Institution. Pawlyn:Amgen, Janssen, Celgene, Takeda: Other: Travel expenses; Amgen, Celgene, Takeda: Consultancy; Amgen, Celgene, Janssen, Oncopeptides: Honoraria. Royle:Celgene, Amgen, Merck, Takeda: Other: Research Funding to Institution. Best:Celgene, Amgen, Merck, Takeda: Other: Research Funding to Institution. Bowcock:Takeda: Honoraria, Research Funding. Boyd:Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Drayson:Abingdon Health: Consultancy, Equity Ownership. Henderson:Celgene, Amgen, Merck, Takeda: Other: Research Funding to Institution. Jenner:Abbvie, Amgen, Celgene, Novartis, Janssen, Sanofi Genzyme, Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Jones:Celgene: Honoraria, Research Funding. Kaiser:Takeda, Janssen, Celgene, Amgen: Honoraria, Other: Travel Expenses; Celgene, Janssen: Research Funding; Abbvie, Celgene, Takeda, Janssen, Amgen, Abbvie, Karyopharm: Consultancy. Kishore:Celgene, Takeda, Janssen: Honoraria, Speakers Bureau; Celgene, Jazz, Takeda: Other: Travel expenses. Mottram:Celgene, Amgen, Merck, Takeda: Other: Research Funding to Institution. Owen:Janssen: Other: Travel expenses; Celgene, Janssen: Consultancy; Celgene, Janssen: Honoraria; Celgene: Research Funding. Jackson:Celgene, Amgen, Roche, Janssen, Sanofi: Honoraria. Cook:Celgene, Janssen-Cilag, Takeda: Honoraria, Research Funding; Amgen, Bristol-Myers Squib, GlycoMimetics, Seattle Genetics, Sanofi: Honoraria; Janssen, Takeda, Sanofi, Karyopharm, Celgene: Consultancy, Honoraria, Speakers Bureau.
Frailty adjusted dosing. Ixazomib and lenalidomide combination as maintenance.
Author notes
Asterisk with author names denotes non-ASH members.