Jing Li, Li Bao and Zhong-jun Xia contributed equally to this study.
Background: Ixazomib (ixa) is the first oral proteasome inhibitor that approved for the use in patients with relapsed/refractory multiple myeloma (RRMM) in > 60 countries. In a recently reported long-term result of a phase 1/2 study (NCT01217957), the all-oral triplet regimen of ixazomib plus Rd (IRd) demonstrated favorable efficacy with acceptable toxicity in patients with newly diagnosed MM (NDMM). Meanwhile, a large phase 3 trial (TOURMALINE-MM2, NCT01850524) evaluating IRd in stem-cell transplantation (SCT) ineligible NDMM patients is ongoing. However, outcomes and toxicity profiles of novel-agent-based MM therapies in real world practice often differ from data reported in clinical trials and data of the efficacy of ixa-based treatment in NDMM in routine practice is currently missing.
Aims and Methods: To assess the efficacy and safety profile of ixa-based frontline therapy in NDMM patients in routine practice, we performed a large national, multi-center, observational study enrolling ixa-treated (at least one cycle completed) NDMM patients from 14 China centers. Clinical records on demographics, disease characteristics, treatment regimen and duration, response rate, adverse events (AEs), and treatment discontinuations and survival were collected and analyzed.
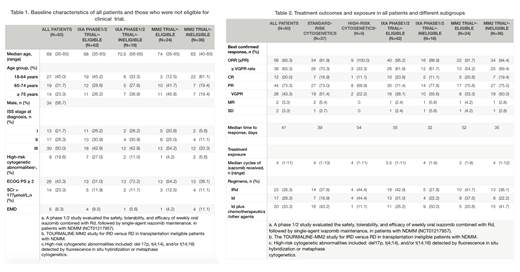
Results: A total of 60 NDMM patients treated with ixa-based regimens were included. Ixa-based regimens included IRd in 23 (38.3%) patients, the ixa and dexamethaxone (Id) in 17 (28.3%) patients and Id plus chemotherapeutics/other agents (Adriamycin in 12 patients, cyclophosphamide in 5 patients, and thalidomide in 3 patients) in 20 (33.3%). None of the patients included received SCT during follow-up.
Median age was 69 years (range 35 - 85) with 33 (55.0%) ≥65 years. At initial diagnosis, ISS stage I/II/III disease were presented in 21.7%/28.3%/50.0% patients at initial diagnosis; high-risk cytogenetic abnormalities (including del 17p, t(4;14), and/or t(14;16) detected by fluorescence in situ hybridization) were detected in 9 patients (19.6%, among 46 patients with FISH results). Twenty-six (43.4%) patients had a ECOG PS ≥2 and 5 patients (8.3%) had extramedullary disease. Eighteen patients were not eligible for ixa phase 1/2 study (NCT01217957) according to its inclusion and exclusion criteria, and even more patients (36, 60%) were not eligible for TOURMALINE-MM2 study. (Table1).
The best confirmed ORR (partial response or better) for all 60 patients was 93.3% (56/60), including 63.3% of patients with ≥VGPR and 20.0% with a CR. The median time to response was 41 days. Similar response was observed among different subgroups: the ORR in Ixa phase1/2 study-eligible/ineligible group, MM2 trial- eligible/ineligible group and patients with standard/high-risk cytogenetics was 95.2%, 88.9%, 91.7%, 94.4%, 91.9% and 100.0%, respectively. And no significant difference in response between different ixa-based regimens was observed. After a median follow-up of 137.5 days after the first dose of ixazomib treatment (range, 28 - 372), median overall survival (mOS) and progression-free survival (mPFS) were not reached. (Table2)
Adverse events (AEs) of grade 3 or higher were uncommon, reported in 14 (23.3%) patients, including thrombocytopenia (4 patients, 6.7%), diarrhea (5 patients, 8.3%), pneumonia (3 patients, 5.0%) and hypokalemia (1, 1.7%). No drug-related grade 3/4 peripheral neuropathy was recorded. Median cycles of ixa received were 4 cycles (range 1-11); 50 (83.3%) were still on treatment at data cut-off; 6 (10.0%) patients discontinued ixa due to intolerable AEs and 4 (6.7%) stop treatment for other reasons (mostly economic concerns).
Discussion and conclusion: Here we reported the first real world, multi-center data on the efficacy and safety profile of ixa-based frontline therapy in patients with NDMM. Our results show that the ixa-based frontline therapy in real-life clinical practice is highly effective and fast in response, with an efficacy data (ORR 93.3%, ≥VGPR rate 63.3%) even better than that reported in NCT01217957 trial (ORR 88.0%, ≥VGPR rate 58.8%). Given the fact that no patients received SCT during follow-up in our cohort, our results maybe more comparable to the ongoing MM2 trial assessing SCT-ineligible NDMM. Ixa-based frontline therapy is well tolerated in NDMM patients treated in routine clinical practice.
No relevant conflicts of interest to declare.
Ixazomib is an oral proteasome inhibitor that approved for the use in patients with relapsed/refractory multiple myeloma (RRMM). Here in this abstract, I will present data on real-life practice of the use of ixazomib in newly diagnosed multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.