Introduction: In 8/8 matched unrelated donor (UD) hematopoietic cell transplantation (HCT), permissive HLA-DPB1 (DP) mismatches within the same functional T Cell Epitope (TCE) group are associated with better outcomes compared to non-permissive mismatches across different TCE groups (Fleischhauer, Blood 2017). This clinical advantage has been shown to be associated with limited in vitro T cell alloreactivity (Meurer, Front Immunol 2019), which in turn is dependent on polymorphic peptide contact amino acids in the DP molecule (Crivello, Biol Blood Marrow Transplant 2015). The HLA class II immunopeptidome is shaped by the peptide editor HLA-DM (DM), and its natural antagonist HLA-DO (DO). Here we investigated the effect of DM/DO activity on the DP immunopeptidome, the breadth of the overall alloresponse to and immunogenicity of permissive and non-permissive DP mismatches, in healthy individuals and in patients after UD-HCT.
Methods: HeLa cells expressing single DP alleles in the presence or absence of DM, or in the presence of DM and DO (Rutten, BBMT 2008), were generated for HLA-DPB1*04:02 (DP4) and *10:01 (DP10) as prototypes for 2 distinct TCE groups. The DP immunopeptidomes were analyzed by mass spectrometry. Alloresponses against DP were quantified by CD137 up-regulation assays after co-culture of irradiated HeLa cells with CD4+ responder T cells from 14 healthy blood donors permissive to DP4 and non-permissive to DP10, or from 2 patients referring to the University Hospital Essen, Germany, the latter alive and well >9 months after 8/8 matched UD-HCT with a permissive DP4 or a non-permissive DP10 mismatch, respectively. The breadth of the responding T cell receptor beta (TCRb) repertoire was determined by immunosequencing (Adaptive Biotechnologies, Seattle, USA). The study was performed under informed consent according to the declaration of Helsinki.
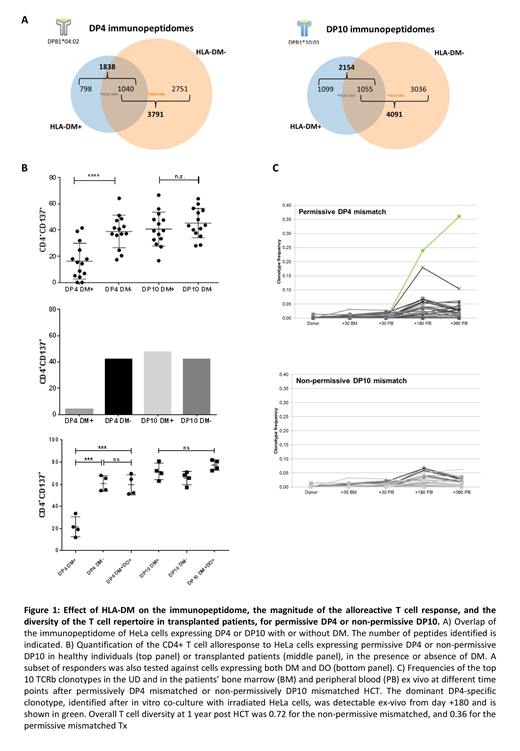
Results: Reflecting their association with different TCE groups, DP4 and DP10 presented peptidomes with limited (<4%) overlap and different peptide motifs. These features were not changed by the presence or absence of DM. In contrast, the presence of DM resulted in a significant (>50%) shrinking of the peptide repertoire displayed by the same DP antigen in the absence of DM, with approximately 30% peptides shared by the same allele in the two conditions, both for DP4 and for DP10 (Figure 1A). In the presence of DM, the magnitude of the T cell alloresponse to non-permissive DP10 was significantly higher than to permissive DP4, both in healthy individuals (40.7% vs 16.3%, respectively, p<0.0001) and in the informative transplanted patients (Figure 1B). Neither the absence of DM (40.7% vs 45.3%, p=ns) nor the presence of DM with DO (71.6% vs 77.4%, p=ns) altered the magnitude of the non-permissive alloresponse to DP10. Compellingly, both the absence of DM (16.3% vs 39.0%, p<0.001) and the co-expression of DM and DO (21.6% vs 59.5%, p<0.001) significantly increased the response to permissive DP4, again both in healthy individuals and in the informative transplanted patients. The strength of the overall alloresponse was associated with the breadth of the corresponding TCRb repertoire, with significantly higher diversity (1-clonality) in response to non-permissive DP10 (mean 0.68) compared to permissive DP4 (mean 0.48) in the presence of DM, and similar high diversity against both DP antigens in its absence (mean 0.74 vs 0.75 against DP4 and DP10, respectively) in healthy individuals. In the transplanted patients, the permissive alloresponse to DP4 was dominated by a single TCRb that could be retrieved at high frequency also in ex-vivo follow-up samples from the same patient from day +195 and +363, while the non-permissive alloresponse to DP10 was polyclonal (mean 0.62 and 0.61 in the presence and absence of DM, respectively) (Figure 1C).
Conclusion: Permissiveness of HLA-DPB1 TCE mismatches is dependent on the peptide editing by DM, and converted into non-permissiveness in its absence or in the presence of its antagonist DO. Permissiveness is associated with the immunopeptidomes of mismatched HLA-DP alloantigens on the MHC side, and with TCRb diversity on the alloreactive T cell side, both in healthy individuals and in patients after UD-HCT. These new mechanistic insights suggest that expression of DM and DO by leukemia or healthy tissues might modulate graft-versus-leukemia and graft-versus-host disease after permissively DP mismatched UD HCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.