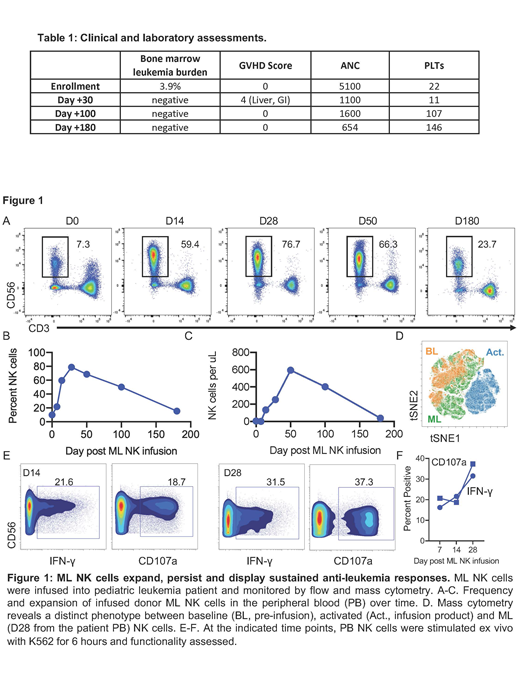
Acute myeloid leukemia (AML) accounts for 18% of pediatric leukemias. For high-risk AML, standard treatment includes multi-agent chemotherapy followed allogeneic hematopoietic cell transplantation (HCT). Despite a high remission rate, 50% of patients relapse and have a poor prognosis with < 20% of patients surviving more than 3 years. Salvage chemotherapy alone or combined with donor lymphocyte infusions (DLI) have little curative potential, and new treatment strategies are needed for relapsed-refractory AML. Previous studies have shown that natural killer (NK) cells can be stimulated ex vivo with IL-12/15/18 to generate a memory-like phenotype with enhanced anti-leukemia effect. In adults with relapsed-refractory AML, adoptive transfer of MHC-haploidentical cytokine-induced memory-like (CIML or ML) NK cells induced remission in 54% of patients (PMID27655849). The infused donor ML NK cells expand in vivo but are rapidly eliminated following recovery of recipient T cells, providing a window of therapeutic activity of 2-3 weeks. We sought to test the safety and efficacy of ML NK cells for treatment of pediatric/young adult patients with post-HCT relapsed AML. We hypothesized that ML NK cells derived from the HCT donor would be well-tolerated, exhibit anti-leukemia activity, and expand with prolonged persistence following transfer into pediatric AML patients. Here, we report the results of the first pediatric patient treated on a phase I clinical trial using ML NK cell therapy for relapsed AML after allogeneic HCT (NCT03068819). Briefly, patients are treated with FLAG (fludarabine, cytarabine and granulocyte colony stimulating factor) salvage chemotherapy to reduce the bulk of AML and provide lymphodepletion for ML NK cell expansion. Two weeks after chemotherapy, a non-mobilized leukapheresis product is collected from the original HCT donor and processed into a T cell-based DLI and ML NK cells. The T cell DLI (1 x 106 T cells/kg) is immediately infused, and the ML NK cells are generated by stimulation with IL-12/15/18 ex vivo for 12-16 hours and then infused (10x106/kg). An 18-month-old male with infant AML had relapse of his leukemia 3 months after MHC-haploidentical HCT. Treatment with chemotherapy, including mitoxantrone and daunorubicin-cytarabine liposome, failed to induce remission. At the time of enrollment on the phase I trial, he had AML blasts in his bone marrow (Table 1). He was treated with FLAG chemotherapy followed by infusion of DLI and ML NK cells from the original haploidentical HCT donor. Assessment at 30 days, 3 months and 6 months post NK cell infusion showed complete remission with no evidence of leukemia and full donor engraftment. Remarkably, donor-derived ML NK cells expanded to 77% of donor lymphocytes on day 28 and still comprised 24% percent of lymphocytes at 6 months post infusion (Figure 1A-C). The expanded donor NK cell phenotype was consistent with ML NK cells (e.g., NKG2A+KIR+) utilizing CyTOF multidimensional analysis previously confirmed to identify ML NK cells (Figure 1D). The ML NK cells were functional as demonstrated by leukemia-triggered IFN-γ production immediately ex vivo from day 7-28 samples (Figure 1E-F). The patient's clinical course was complicated by mild gastrointestinal graft-versus-host disease that resolved with low-dose steroids and tociluzimab. These early results demonstrate proof-of-principle that adoptive transfer of donor-derived ML NK cells in combination with DLI is feasible and offers a novel immunotherapy option for patients with relapsed AML after HCT. Moreover, in this T and NK cell compatible immune environment post-HCT, donor ML NK cells expand and persist robustly in vivo for > 6 months without exogenous cytokine support and have potent anti-leukemic activity. Thus, ML NK cells are a cellular therapy platform to treat AML that has relapsed after allogeneic HCT.
Cashen:Celgene: Other: Speaker's Bureau; Seattle Genetics: Other: Speaker's Bureau; Novartis: Other: Speaker's Bureau. Fehniger:Horizon Pharma PLC: Other: Consultancy (Spouse); Cyto-Sen Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.