Objective: Relapsed refractory multiple myeloma (RRMM) is a chronic disease characterized by multiple relapses and lines of therapy. Patients may experience severe symptom burden that impacts treatment adherence. Digital PRO reporting and symptom management in response has shown improved survival and adherence as well as maintenance of health-related quality of life in patients with solid-tumors. Evidence for the efficacy of a similar approach is lacking for patients with RRMM. This implementation study was designed to test the acceptability, appropriateness, and understandability of digital PRO reporting for RRMM patients, and explore its impact on clinic workflow and symptom management. The results are intended to inform and provide guidance for future iterations of digital PRO reporting initiatives for this, or a similar, patient population.
Methods: A selective sampling strategy was used to recruit 11 patients with RRMM who had received between 1 and 3 lines of therapy, and were receiving treatment at the John Theurer Cancer Center in Hackensack, NJ. The study period was 3 weeks.
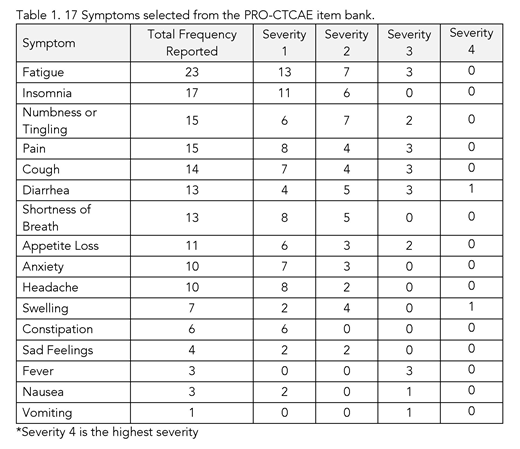
Patients reported on the presence, severity, and interference of 17 symptoms from the PRO-CTCAE (Table 1) at 4 scheduled sessions, each a week apart via a digital app. Patients could also report symptoms spontaneously. When symptom reports met pre-defined thresholds, through the app patients received automatically populated symptom self-management guidance, specified by the clinic, and providers were alerted. Reporting details, such as symptom selection and alert criteria, were defined by the clinic team prior to study initiation.
Patient experience was assessed through 2 semi-structured interviews conducted approximately 2 weeks apart, a 2-item perceived ease-of-use and understandability measure, and app usage data. Provider experience was assessed through a semi-structured group interview, a 6-item acceptability survey, and abbreviated clinic workflow observation.
Results: Nine patients (mean age = 69.7 years) completed the study; 55% were female. Mean time since diagnosis was 10.9 years.
Overall, 83% of weekly sessions were completed. All patients rated the app as very easy to use and understandable. All patients found the list of symptoms tracked to be appropriate and the reporting frequency to be acceptable. All patients reported preferring the mobile app over the web portal. Neither patients nor providers reported viewing the symptom reports, which displayed symptom reports over time. Patients reported benefitting from symptom reporting through increased awareness of their symptoms.
A total of 33 symptom alerts were generated. Phone calls to the clinic related to reported symptoms increased during the study period. The majority of the providers reported that it was difficult to respond to alerts because of lack of integration with their current system. Providers did not find additional value in receiving symptom alerts from patients between clinic visits because many of those symptoms had previously been addressed through existing communication channels. Patients reported some redundancy in clinic outreach responses to an alert. They perceived that their reported symptoms may have already been addressed, or were not significant enough to warrant a response. No provider discussed symptoms reported via the app at clinic visits.
Conclusions: RRMM patients found the app content appropriate, acceptable, and understandable. To increase the value of digital PRO reporting for RRMM symptom management, the clinic team recommended modifications that focus on addressing "treatment fatigue" and controlling patients' long-term disease- and treatment-related symptoms. This could be achieved through the use of longitudinal symptom reports to create more structured, efficient, and meaningful conversations during clinic visits. To increase adoptability, providers felt the symptom alert triggers required revision to make them more clinically meaningful, avoid redundancy, minimize impact on the clinic workflow, and increase integration with existing clinic systems. The results of this implementation study support that digital PRO reporting is acceptable to RRMM patients, and that after implementing modifications, it could be helpful to improve the quality of patient-provider communication, increase adherence, and duration on therapy.
Biran:Takeda: Consultancy, Honoraria; Bristol Meyers Squibb: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Merck: Research Funding. Yucel:Amgen Inc.: Employment, Other: Owns Amgen stock, Research Funding. Anthony Kouyaté:Amgen: Employment, Other: Owns Amgen stock. McGovern:Amgen: Consultancy. Schoenthaler:Rip Road: Consultancy. Durling:Amgen: Consultancy. Schutt:Amgen: Consultancy. Panjabi:Amgen Inc.: Employment, Other: Owns Amgen stock, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.