Sickle Cell Disease (SCD) is characterized by hemolytic anemia, vaso-occlusion, and progressive end-organ damage. The underlying mechanism of SCD is the polymerization of sickle hemoglobin (HbS) that occurs when sickle erythrocytes (SS RBCs) are partially deoxygenated in microcirculation, leading to SCD pathophysiologic features. One of the most devastating complications of SCD occurs in the central nervous system (CNS), where overt stroke or repeated silent cerebral infarcts lead to significant physical and neurocognitive consequences. In SCD, the brain's response to insufficient oxygen (O2) delivery is balanced by increased blood flow to preserve O2 supply. However, the systemic endothelial dysfunction in SCD limits the capacity for vascular regulatory and compensatory changes to preserve appropriate tissue oxygenation, especially in tissues with high O2 demand like the brain. Low hemoglobin (Hb) levels and increased cerebral blood flow (CBF) are associated with increased stroke risk, suggesting that anemia-induced tissue hypoxia is an important factor contributing to subsequent morbidity in SCD patients.
Voxelotor (GBT440) is a small molecule, HbS polymerization inhibitor being developed by Global Blood Therapeutics (GBT) for the treatment of SCD. By addressing the underlying mechanism of SCD, voxelotor has the potential to be disease-modifying and alleviate the clinical manifestations of SCD. Mechanistically, voxelotor increases Hb-O2 affinity and delays the transition from oxyHb to deoxyHb under hypoxic conditions.
This study assessed the impact of a pharmacologically mediated increase in Hb-O2 affinity on brain tissue oxygenation under both normoxic and hypoxic conditions in Townes transgenic sickle mice (SCD mice). Two compounds that increase the Hb-O2 affinity with similar potency, voxelotor and an analog to voxelotor, GBT1118, were considered for the study. The target for Hb occupancy with test compounds was ≥30% based on the therapeutic target occupancies observed with voxelotor in clinical studies. The effects of increased Hb-O2 affinity on brain tissue oxygenation were assessed directly with O2-specific microelectrodes in a cranial window and indirectly with hypoxyprobe staining (pimonidazole) of brain tissue. Unique to this SCD model, the targeted Hb occupancy (≥30%) could not be consistently achieved by voxelotor. In contrast, repeat oral dosing of GBT1118 at 200 mg/kg/day for 2 weeks in SCD mice achieved steady state concentrations of 802 ± 81 µM (mean ± SD; n=5), corresponding to a Hb occupancy of 44 ± 5%. Consequently, GBT1118 decreased the p50 (partial pressure of O2 at which Hb is 50% saturated) values of SCD mouse blood from 39 ± 0.8 mmHg (Vehicle-dosed) to 21 ± 1.6 mmHg (GBT1118-dosed). While we could not achieve the desired Hb occupancy (≥30%) with voxelotor in this model, the Hb occupancy and change in p50 achieved with GBT1118 afforded us the opportunity to ask whether significantly increasing Hb-O2 affinity affects brain O2 tension.
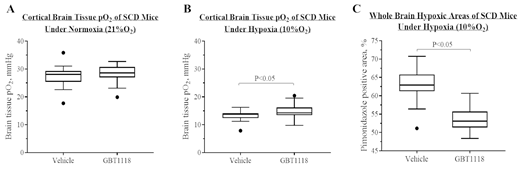
Measurements of cortical O2 tension (pO2) showed no difference in pO2 values under normoxia (21% O2) (Figure A), and slightly higher pO2 values under hypoxia (10% O2) (Figure B) for GBT1118-dosed SCD mice compared with vehicle-dosed SCD mice. Collectively across all brain tissues, the GBT1118-induced increase in Hb-O2 affinity reduced tissue hypoxia in SCD mice under hypoxia as measured by pimonidazole staining (Figure C). Together, these results indicate that a pharmacological increase of Hb-O2 affinity does not decrease cortical tissue pO2 in SCD mice and may reduce brain hypoxia under hypoxic conditions.
Dufu:Global Blood Therapeutics: Employment, Equity Ownership. Lucas:Global Blood Therapeutics: Research Funding. Muller:Global Blood Therapeutics: Research Funding. Williams:Global Blood Therapeutics: Research Funding. Zhang:Global Blood Therapeutics: Employment, Equity Ownership. Rademacher:Global Blood Therapeutics: Employment, Equity Ownership. Alt:Global Blood Therapeutics: Employment, Equity Ownership. Oksenberg:Global Blood Therapeutics: Employment, Equity Ownership. Cabrales:Global Blood Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.