Background: Coordinated interactions between neutrophils, platelets and endothelial cells contribute towards the development of arterial thrombosis. Neutrophils along with platelets are the first immune cells that are recruited at the site of endothelial activation/injury or infection. Recent studies have suggested that neutrophils modulate thrombosis via several mechanisms, including NETosis (formation of neutrophil extracellular traps). The integrin α9 is highly expressed on neutrophils while platelets do not express it. The integrin α9 up-regulated upon neutrophil activation and is implicated in stable adhesion and transmigration. The mechanisms underlying the role of integrin α9 towards the progression of arterial thrombosis has not been explored yet.
Objective: To elucidate the mechanistic insights into the role of myeloid-cell specific integrin α9 in neutrophil adhesion and arterial thrombosis.
Methods: We generated novel myeloid-specific α9-/- mice (α9fl/fl LysMcre+l-) by crossing α9fl/fl with LysMcr+/+mice. Littermates α9fl/flLysMcre-l-mice were used as controls. Standardized in vitro assays were used to evaluate the role of integrin α9 in neutrophil mediated platelet aggregation, NETosis and Cathepsin-G release. Susceptibility to arterial thrombosis and hemostasis was evaluated in vivo (FeCl3-induced carotid and laser-injury induced mesenteric artery thrombosis models) by utilizing intravital microscopy and tail bleeding assay respectively.
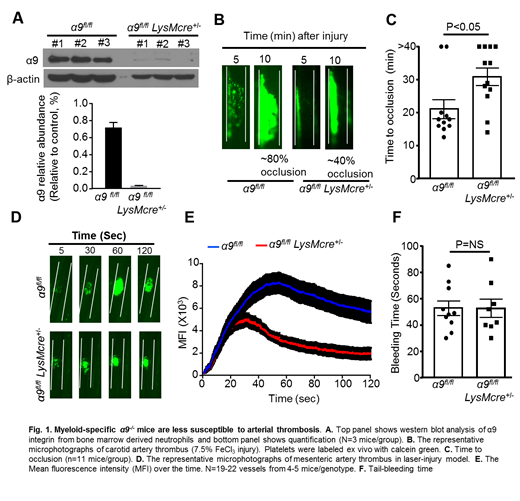
Results:α9fl/flLysMCre+/-mice developed smaller thrombi (~40% occlusion), when compared with α9fl/flmice (~80% occlusion, 10 minutes post-FeCl3 induced injury). The mean time to complete occlusion was significantly prolonged in α9fl/flLysMCre+/-mice (P<0.05 vs α9fl/fl mice). Consistent with this, α9fl/flLysMCre+/-mice displayed significantly decreased platelet mean fluorescence intensity (MFI) and reduced rate of thrombus growth in laser injury-induced thrombosis model (P<0.05 vs. α9fl/fl mice). Together, these results suggest that myeloid cell-specific integrin α9 contributes to the experimental thrombosis at arterial shear rates. Monocytes depletion experiments demonstrated a minimal role for monocyte in progression of arterial thrombosis. In vitro mechanistic studies demonstrated a reduction in neutrophil-mediated platelet aggregation and cathepsin-G secretion in myeloid cell-specific integrin α9-/- mice, when compared with litter-mates control wild-type mice. Notably, the percentage of cells releasing NETs was markedly reduced in myeloid cell-specific integrin α9-/- mice that was concomitant with reduced MPO levels in carotid thrombus of α9fl/flLysMCre+/-mice. Together, these results suggest most likely integrin α9 expressed on neutrophils, but not monocytes, promotes arterial thrombosis. Comparable tail bleeding time between α9fl/flLysMcreand littermate α9fl/fl mice suggested that myeloid-cell specific deficiency of integrin α9 does not alter hemostasis.
Conclusion: These findings reveal a novel role for integrin α9 in modulation of arterial thrombosis. While the clinical implications of these findings remains to be explored, we suggest that targeting integrin α9 may reduce post reperfusion thrombo-inflammatory injury, following acute myocardial infarction or stroke.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.