Currently, it is not clear why some patients with acute myeloid leukemia (AML) can be "cured" with chemotherapy alone; are they living with small amounts of disease that is held in check by immunologic (or other) mechanisms, or is their disease really eradicated? The percentage of cytogenetically normal AML patients who have long (>5 years) first remissions (LFRs) after chemotherapy alone is low (about 9.1% in patients <60 years and 1.6% in >60 years1). For this reason, most intermediate risk patients are offered allogeneic transplantation to decrease their risk for relapse.
To better understand mechanisms of chemotherapy sensitivity in AML, we performed an analysis of the mutation landscape and persistence, using samples from 8 normal karyotype LFR patients (without CEBPA mutations) who received standard "7+3" induction and high dose cytarabine consolidation as their only therapy. The mean age at diagnosis was 43.5 years, and the mean follow up in first remission is 7.6 years; none of these patients has relapsed to date.
For each case, we performed enhanced exome sequencing at diagnosis (235x coverage of the entire exome, and ~1008x coverage of recurrently mutated AML genes). Each case had at least one documented AML driver mutation, with a median of 29 somatic mutations in the exome space. We created probes for 225 mutations (mean 28 per case), and performed error-corrected sequencing (Haloplex) for all available remission samples. The mean depth of Haloplex coverage was 1607x, and each sample had at least one AML-specific mutation assayed, with a sensitivity of 1 cell in 1,750 (0.06%).
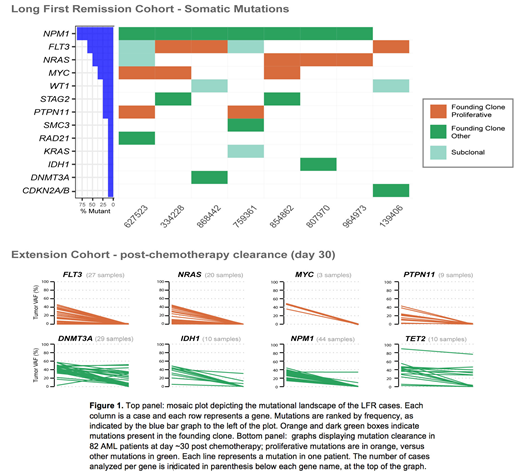
7/8 patients demonstrated complete clearance of all mutations in all remission samples tested, which was confirmed with digital droplet PCR for 5 cases, with a sensitivity of detection of 1 cell in 100,000. In one case, we detected a persistent ancestral clone harboring DNMT3AR882H, which can be associated with long first remissions for some patients2. Strikingly, the founding clone in all 8 cases had one or more somatic mutations in genes known to drive cell proliferation (e.g. MYC, FLT3, NRAS, PTPN11, Figure 1 top panel). These are usually subclonal mutations that occur late during leukemic progression, suggesting that the presence of a "proliferative hit" in the founding clone might be important for chemotherapy clearance of all the AML cells in a given patient. To support this hypothesis, we analyzed the mutational clearance of 82 AML cases with paired diagnosis and day 30 post-chemotherapy bone marrow samples. We observed that, whether present in the founding clone or in subclones, mutations in MYC, CEBPA, FLT3, NRAS, and PTPN11 cleared after induction chemotherapy in all samples, while other mutations were often persistent at day 30 (e.g. DNMT3A, IDH1, IDH2, NPM1, TET2; Figure 1 bottom panel). Compared to other published sequencing studies of AML, MYC and NRAS mutations were significantly enriched in this small cohort (MYC p= 0.002, and NRAS p= 0.034), with MYC enrichment being particularly striking (37.5% versus 1.8%). All MYC mutations were canonical single base substitutions occurring in the highly conserved MYC Box 2 domain at the N-terminus of MYC (p.P74Q or p.T73N).
Overexpression of MYCP74Q in murine hematopoietic progenitors prolonged MYC half life (89 min vs. 44 min for wild type), and enhanced cytarabine sensitivity at all concentrations tested (range 10-1000 nM, p=0.0003), both in vitro and in a MYC-driven leukemia model in vivo. MYC expression measured with flow cytometry in the blasts of the LFR samples was significantly higher (p=0.045) compared to unfavorable risk (complex karyotype) or other intermediate risk categories, but similar to good risk AML (biallelic CEBPA mutations, core binding factor fusion-associated AML, and AML with isolated NPMc), suggesting that activation of the MYC pathway may represent a shared feature of chemosensitive patients.
Taken together, these data suggest that some intermediate patients who are effectively "cured" with chemotherapy alone may not have persistent subclinical disease, nor retained ancestral clones that could potentially contribute to relapse. Importantly, these patients often have mutations driving cell proliferation in the founding clone, indicating that the presence of specific mutations in all malignant cells may be critical for complete AML cell clearance with chemotherapy.
1. Blood Adv. 2018 Jul 10; 2(13): 1645-1650
2. N Engl J Med 2018; 378:1189-1199
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.