Background:
Outcomes of patients with AML have remained poor despite the availability of cytotoxic chemotherapy, hypomethylating agents (HMAs) and targeted therapies. HMAs, such as azacitidine, in combination with Bcl-2 inhibitors like venetoclax have demonstrated response rates of 67% in newly diagnosed AML and 21% in relapsed/refractory (RR) AML (DiNardo et al. Blood 2019 and Am J Hematol 2018). While the combination of azacitidine and venetoclax is efficacious in AML, preclinical studies indicate potential mechanisms of drug resistance including overexpression of MCL-1, an anti-apoptotic protein (Konopleva et al. Cancer Cell. 2006). Pevonedistat is a first in class inhibitor of Nedd8 activating enzyme that has demonstrated activity against AML (Swords RT et al. Blood. 2010). Pevonedistat induces NOXA, a pro-apoptotic protein leading to neutralization of MCL-1 inducing apoptosis (Wang et al. Biochem Biophys Res Commun. 2017). Preclinical studies evaluating the combination of pevonedistat and venetoclax against AML cell lines have demonstrated synergistic effect (Knoor KL et al. Cell Death Differ. 2015). Hence, we hypothesize that the addition of pevonedistat to the combination of azacitidine and venetoclax would enhance the therapeutic efficacy by overcoming resistance to apoptosis.
Study design and methods:
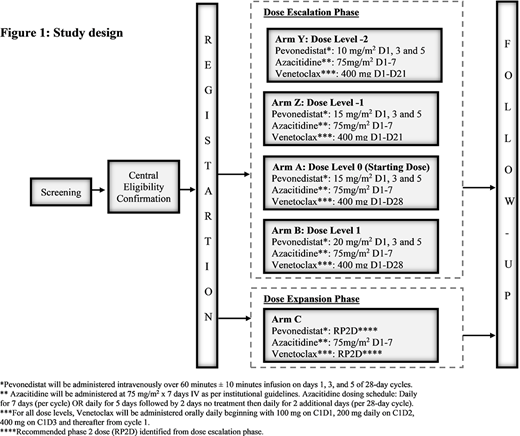
This is an investigator-initiated phase Ib study evaluating the safety of pevonedistat, azacitidine and venetoclax. Patients aged 18 years or above with morphologically documented AML (de novo, secondary or therapy-related), ECOG performance status 0-2 and adequate organ function are eligible for the study. Major exclusion criteria are patients with isolated extramedullary relapse, hematopoietic cell transplantation (HCT) within 100 days of enrollment, active acute GVHD, veno-occlusive disease, acute promyelocytic leukemia, liver cirrhosis and severe liver impairment. While the dose escalation phase is available only for patients with RR-AML, the dose expansion phase can also include newly diagnosed AML patients who are ineligible for intensive induction. The study is planned to be conducted at Medical College of Wisconsin, Mayo Clinic and University of Pennsylvania.
The primary endpoint is to determine the recommended phase 2 dose (RP2D) and toxicity profile of pevonedistat, azacitidine and venetoclax. The secondary endpoints include determination of response rates, duration of response, survival and pharmacokinetics. Exploratory endpoints include correlation of response rates with AML genomic profile, correlation of pretreatment levels of BCL2, BCLXL, MCL1, BAX or BAK with response, determination of changes in NOXA (PMAIP1) mRNA and protein expression pre-and post-pevonedistat treatment, evaluation of BH3 mimetic profiling on bone marrow samples by flow cytometry and assessing the sensitivity of leukemia and leukemic stem/progenitor cells to pevonedistat ex vivo.
The study will follow 3+3 design with dose escalation (Arms A and B), de-escalation in case of dose limiting toxicity (DLT) (arms Z and Y) and dose expansion phase (figure 1). Patients will be entered sequentially to each dose level, starting with dose level 0. The DLT observation period for dose-escalation will be 1 cycle. The maximal tolerated dose (MTD) will be defined as the highest dose level at which none of the first 3 treated subjects, or no more than 1 of the first 6 treated subjects experiences a DLT. A minimum of 9 and a maximum of 24 patients will be needed for the dose escalation phase and 6 patients for the dose expansion phase. Response rate, duration of response and exploratory endpoints will be analyzed using descriptive statistics. Kaplan-Meier method will be used to determine survival.
Guru Murthy:Cardinal Health Inc.: Honoraria. Michaelis:Incyte: Consultancy, Research Funding; Pfizer: Equity Ownership, Research Funding; Novartis: Consultancy; Macrogeneics: Research Funding; Millenium: Research Funding; BMS: Research Funding; Celgene: Consultancy, Research Funding; JAZZ: Other: Data Safety Monitoring Board, uncompensated, Research Funding; TG Therapeutics: Consultancy, Research Funding; Janssen: Research Funding; ASTEX: Research Funding; Bioline: Research Funding. Abedin:Jazz Pharmaceuticals: Honoraria; Agios: Honoraria; Helsinn Healthcare: Research Funding; Pfizer Inc: Research Funding; Actinium Pharmaceuticals: Research Funding. Runaas:Agios: Honoraria; Blueprint Medicine: Honoraria. Atallah:Jazz: Consultancy; Novartis: Consultancy; Takeda: Consultancy, Research Funding; Pfizer: Consultancy; Jazz: Consultancy; Helsinn: Consultancy; Helsinn: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.