Background: Marginal zone lymphoma (MZL) is an incurable but heterogeneous disorder for which there is no standard of care treatment in the relapsed/refractory (R/R) setting. The BTK inhibitor, ibrutinib (IB) is well tolerated with an overall response rate (ORR) of 48% as monotherapy in R/R MZL but rarely complete remission (Noy et al, Blood, 2017). The BCL-2 inhibitor, venetoclax (VEN) has also been evaluated in a small number of R/R MZL patients (pts) with evidence of activity and tolerability (Davids et al, JCO, 2017). We sought to evaluate the efficacy and safety of combination IB and VEN in pts with MZL based upon the rationale of (1) distinct mechanisms of action, (2) activity as monotherapies in MZL, and (3) acceptable non-overlapping toxicity profiles. We report preliminary results of the MZL cohort of an investigator-initiated phase II trial of combination IB and Ven (NCT02471391).
Methods: Pts with MZL by WHO 2008 criteria who were R/R or treatment naïve but considered inappropriate for treatment with chemotherapy were enrolled. Pts commenced treatment with IB monotherapy at a dose of 560mg per day. After 4 weeks, VEN was added to IB treatment, in weekly step-wise dose escalation over 6 weeks to a target dose of 800mg per day in the initial cohort (n = 4), amended to 400mg per day, due to a reported IB-VEN drug interaction in a similar trial (NCT02910583). The primary endpoint was the complete remission rate (CRR) at 16 weeks. The secondary endpoints were to determine ORR and CRR, to determine minimal residual disease (MRD) elimination rates, to describe progression free survival (PFS), overall survival, duration of response, time to progression and frequency and severity of adverse events. Investigator-assessed response assessments, based on IWG criteria (Cheson et al, JCO, 2007), were performed with CT at 4, 16, 28, 40 and 56 weeks, PET/CT at weeks 16 and 56, and bone marrow (BM) aspirate and trephine, and MRD assessments by flow cytometry at all time-points.
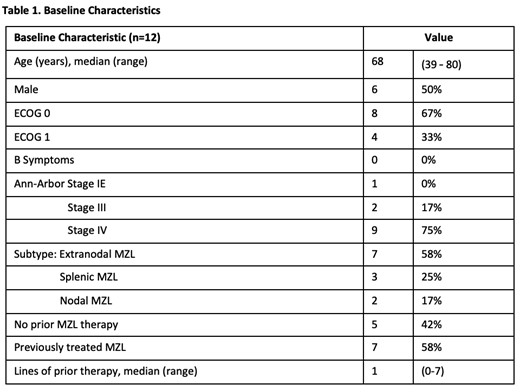
Results: Fourteen of 15 planned pts were enrolled at the time of data cut-off (May 2019), of whom 12 had reached the week 16 primary endpoint or discontinued and are reported here. Two remain on study for <16 weeks at data cut-off. The median age was 68 years (range, 39 - 80yrs). Baseline characteristics are shown in Table 1. Seven pts (58%) had R/R MZL with median 1 prior therapy; 5 (42%) were treatment naïve. All pts received treatment, completing IB monotherapy and commencing the VEN and IB combination. One pt discontinued treatment during VEN escalation (50mg Ven) due to grade 4 drug-induced hepatitis, attributed to IB following rechallenge. The median follow-up is 9.7 months (range, 3-12 months).
The CRR with CT assessment at week 16 was 25% (3/12). The CRR including PET assessment was 42% (5/12) at week 16. The ORR at this time-point was 58% (7/12) by CT and 84% (10/12) by PET (historical control with IB monotherapy is 3%; Noy et al, Blood, 2017). MRD clearance (10^-4 sensitivity) in BM was confirmed in 1 of 7 evaluable pts.
Two pts have developed PD after 7 and 11 months of treatment commencement. No deaths have occurred.
Safety analysis shows no cases of tumour lysis syndrome, and only one (8%) discontinuation due to toxicity. Grade 1-2 atrial fibrillation (AF) occurred in 2 pts (17%), suspected to be related to IB. The most common non-hematologic toxicities were diarrhea, bruising, fatigue and nausea, and were largely low grade. The most common hematologic toxicities were neutropenia (n=6) and thrombocytopenia (n=5) including grade 3-4 events (2 and 1 events, respectively). Serious adverse events (SAE) occurred in 5 subjects (1 pt had 2 SAE's), including 2 events attributable to trial therapy. Overall 2 pts (17%) required permanent dose reduction of IB to 420mg, due to grade 2 AF and due to grade 3 febrile illness in these cases. One pt (8%) required permanent dose reduction of VEN to 300mg, due to grade 4 neutropenia lasting >7 days despite use of G-CSF.
Conclusions: Combination IB and VEN is a promising safe and efficacious treatment for MZL. Our preliminary data, demonstrating high response rates including frequent CR, warrants further investigation in a larger study.
Handunnetti:Gilead: Honoraria; Abbvie: Other: Travel Grant. Khot:Amgen: Speakers Bureau; Kyowa Hakko Kirin: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Celgene: Consultancy. Anderson:Walter and Eliza Hall Institute: Employment, Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.. Blombery:Novartis: Consultancy; Janssen: Honoraria; Invivoscribe: Honoraria. Ritchie:Sanofi: Honoraria; Novartis: Honoraria; Imago: Research Funding; Beigene: Research Funding; Takeda: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; BMS: Research Funding. Hicks:Telix Pharmaceuticals: Equity Ownership; Ipsen: Research Funding; Clarity: Research Funding. Bressel:Regeneron Pharmaceuticals: Consultancy. Roberts:Australasian Leukaemia and Lymphoma Group: Membership on an entity's Board of Directors or advisory committees; Walter and Eliza Hall Institute: Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.; AbbVie: Other: Unremunerated speaker for AbbVie, Research Funding; Janssen: Research Funding; BeiGene: Research Funding. Seymour:Janssen: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy; Acerta: Consultancy; Roche: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau. Tam:Novartis: Honoraria; Janssen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; BeiGene: Honoraria; Roche: Honoraria; Pharmacyclics LLC, an AbbVie company: Honoraria.
Discussion of off-label use: Venetoclax and combination venetoclax and ibrutinib are not approved in the US for use in marginal zone lymphoma.
Author notes
Asterisk with author names denotes non-ASH members.