Background
Approximately half of patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL) will relapse despite curative intent therapy with salvage chemotherapy (SC) and autologous stem cell transplantation (ASCT). Consolidative radiotherapy (cRT) is used to reduce the risk of local relapse in high risk patients. We aimed to examine the (i) impact of cRT on outcomes, (ii) role of positron-emission tomography computerised tomography (PET-CT) imaging in predicting outcomes, (iii) identify a subgroup most likely to benefit from cRT, (iv) assess patterns of relapse to understand the mechanisms of treatment failure and (v) propose a PET-CT based risk stratification model for relapse.
Patients and Methods
104 patients with R/R cHL treated with curative intent SC and ASCT between January 2000 and May 2019 were retrospectively identified from two sites in Melbourne, Australia. Fifty-six patients (54%) received peri-transplant cRT. SC consisted of chemotherapy and/or immunotherapy of the clinician's choice followed by interim assessment by PET-CT imaging. Patients with a complete or partial metabolic response proceeded to ASCT. Disease status was routinely evaluated by physical examination and PET-CT imaging at time of disease relapse, at completion of salvage chemotherapy, and at three months following ASCT. A complete metabolic response on fluorodeoxyglucose (FDG) PET/CT was defined by Deauville Score ≤3 (uptake ≤ liver, PET negative) and compared to Deauville Score ≥4 (PET positive).
Results
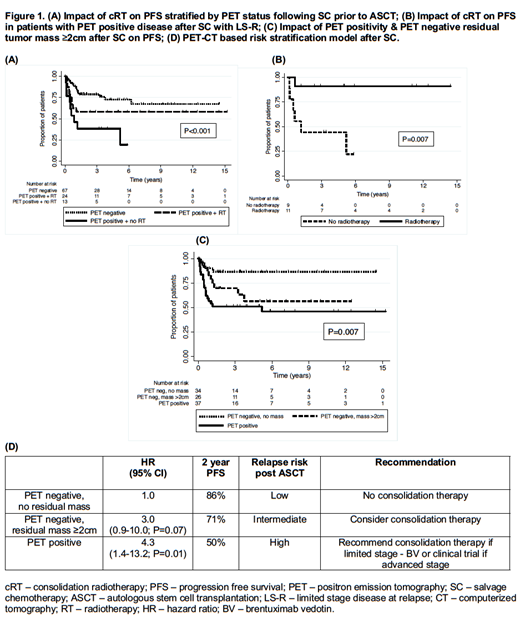
With a median follow-up of 2.5 (range 0.2-15.2) years, the 2-year progression free survival (PFS) and overall survival (OS) rates were 68.3% and 83.6%, respectively. Inferior PFS was associated with advanced stage (III or IV) disease at relapse (AS-R) (53% vs 78%; P=0.004) and PET positivity after SC (51% vs 79%; P=0.005). Compared to PET negative patients after SC, 2-year PFS was significantly inferior in patients who were PET positive and did not receive cRT (38% vs 79%; P<0.001) which lost statistical significance with the addition of cRT (Figure 1A. 58% vs 79%; P=0.12) despite patients in each RT cohort having comparable baseline characteristics. This potential PFS benefit was statistically significant for PET positive patients after SC with limited stage disease at relapse [LS-R] (Figure 1B. 91% vs 44%; P=0.007) but not for patients with AS-R (31% vs 25%; P=0.90). Patients who achieved a negative PET scan without residual tumor mass had superior outcomes following ASCT as compared to patients who are PET negative with residual mass ≥2cm and PET positive patients (Figure 1C. 86% vs 70% vs 51%; P=0.007). Although cRT was associated with a non-significant improvement in local disease in patients with AS-R (P=0.05), 3/5 (60%) patients with LS-R and 5/12 (42%) patients with AS-R who received cRT had subsequent in-field relapse post ASCT.
Conclusion
One third of patients with relapsed/refractory cHL who received SC and ASCT subsequently relapsed within two years. cRT may abrogate the negative predictive value of a positive PET scan after SC, of which patients with LS-R are most likely to benefit. PET positive disease and residual mass ≥2cm after SC are associated with inferior PFS despite the use of cRT. Conversely, patients who are PET negative without residual tumor mass after SC have superior outcomes. cRT is associated with improved local disease control, yet approximately half of patients who relapse after cRT will still have in-field relapse, possibly warranting the evaluation of higher cRT doses. Therefore, we propose a PET-based model which may identify the patients at "high" risk of progressing after ASCT who are most likely to benefit from clinical trials examining different modalities of consolidative therapy (Figure 1D). Validation of this model is required in a larger independent population.
Ritchie:Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; BMS: Research Funding; Takeda: Research Funding; Beigene: Research Funding; Imago: Research Funding; Novartis: Honoraria; Sanofi: Honoraria. MacManus:National Health and Medical Research Council Australia: Research Funding. Seymour:Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Honoraria; Acerta: Consultancy, Honoraria. Dickinson:GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding, Speakers Bureau; Merck Sharpe and Dohme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.