Introduction: Dysregulated C-MYC signaling is associated with the pathogenesis and poor survival in aggressive lymphomas such as diffuse large B cell lymphoma (DLBCL). Despite intense investigation, direct inhibitors of C-MYC have not yet advanced to the clinic. Various structural elements, such as TOP (terminal oligopyrimidine tract) motif, guanine quartet (CGG) 4 motif, and polypurine sequences (AGAGAG), have been shown to impart high level of dependence on eukaryotic initiation factor 4F (eIF4F). The eIF4F is comprised of 3 subunits, including the mRNA 5ʹ-cap-binding subunit eIF4E, the large scaffolding subunit eIF4G, and the RNA helicase subunit eIF4A. In the current project, we have investigated targeting eIF4A as a novel therapeutic strategy in lymphoma. Pateamine A (PatA) is a natural product originally isolated from a New Zealand marine sponge, Mycale sp. DMDA-PatA, a first generation PatA analogue, has demonstrated in vivo activity in xenograft cancer models. DMDA-PatA acts by decreasing the interaction between eIF4A and eIF4G that specifically inhibits cap dependent translation. MZ-735 is a recently developed PatA analogue that is more potent than DMDA-PatA and less protein bound.
Materials and Methods: MZ-735 was synthesized at the CPRIT Synthesis and Drug-Lead Discovery Laboratory at Baylor University. Cytotoxicity was evaluated in diffuse large B cell and Mantle cell lymphoma cell lines using CellTiter-Glo (Promega®). Western blot analysis was performed for the expressions of oncogenes. Global translation was determined using surface sensing of translation (SUnSET) assay and Polysome profile followed by Western blotting and qPCR. RNA sequencing (RNA-seq) was carried out on drug versus dimethyl sulfoxide (DMSO)-treated cells in DLBCL and MCL. An unbiased proteomics analysis was performed using TMT mass spectrometry. Omics data were analyzed by gene set enrichment analysis (GSEA) and Virtual Inference of Protein-activity by Enriched Regulon (VIPER) analysis.
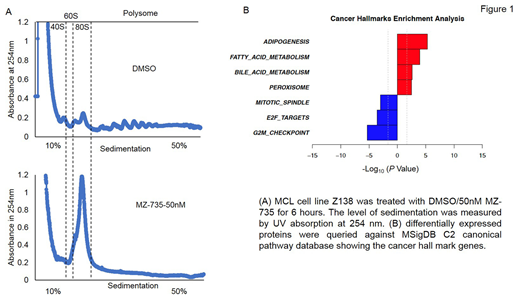
Results: MZ-735 were studied in 13 lymphoma cell lines. MZ-735 exhibited concentration- and time-dependent cytotoxicity in lymphoma cell lines at low nanomolar concentrations and showed minimal toxicity in normal PBMC cells. MZ-735 potently inhibits polysome formation, global protein synthesis and inhibits oncogene such as C-Myc and Cyclin D1 in DLBCL and MCL cells. An unbiased quantitative proteomics analysis was carried out to identify the immediate consequence of MZ-735 during a short exposure time of 3 hours. More than 5000 proteins were detected, of which 132 proteins were upregualated and 119 proteins were down regulated by more than twofold after MZ-735 treatment (P < 0.05; fold change, >2). Enrichment analysis suggested that Cancer hallmark proteins such as E2F target proteins, G2/M check point proteins and mitotic spindle proteins were highly enriched among these depleted proteins and the most affected downregulated pathways are cell cycle, cell division and chromosome segregation pathway. We are currently conducting RNAseq to determine whether the mRNA level change in the same or opposite direction as the protein level for any given gene across the genome. Furthermore, we will conduct proteomics and RNAseq in samples treated for longer time points, as the short exposure (3h) favors discovery of depleted proteins with very short half-lives.
Conclusion: These results demonstrate that the PatA analogue MZ-735 can potently inhibit the translational apparatus in lymphoma. MZ-735 inhibits metabolic pathways required for cancer progression, leading to growth arrest and apoptosis in the lymphoma cells. MZ-735 and other eIF4A inhibitors represent a promising new class of anti-neoplastic agents, which may be particularly useful for silencing undruggable oncogenes such as C-MYC. Deep insights into the translational responses to eIF4A inhibitors at the single gene level will inform future development of this class of anti-neoplastic agents.
O'Connor:Allos Therapeutics: Consultancy; Acetylon Pharma: Other: Travel expenses, Research Funding; Celgene: Research Funding; Millenium: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Mundipharma: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Novartis: Consultancy, Honoraria; Roche: Research Funding; Seattle Genetics, Inc.: Consultancy, Other: Travel expenses, Research Funding; Spectrum Pharma: Consultancy, Other: Travel expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.