Introduction
The clinical characteristics, treatment, cardiovascular events (CVE) and evolution of patients diagnosed with JAK2 V617F positive essential thrombocythemia (ET) with low allele burden (LAB) are scarcely studied. Its presence in people without a confirmed diagnosis of malignant hemopathy is called clonal hematopoiesis of uncertain significance (CHIP) and confers higher risk of developing CVE. The objective of this study was to compare the clinical characteristics and CVE of a series of JAK2 V617F-positive ET patients with <10% (LAB) vs. ≥10% allele burden (HAB), from the GEMFIN (Grupo Español de Enfermedades Mieloproliferativas Crónicas Filadelfia Negativas) Group.
Methods
From the database of the GEMFIN group, 410 ET patients were JAK2 V617F positive, 89 (21.7 %) with LAB and 321 (78.3%) with HAB. The clinical characteristics, treatment (cytoreduction, antiagregation, anticoagulation, JAK inhibitor), CVE (before, at and after diagnosis) and evolution to myelofibrosis (MF) or acute myeloid leukemia (AML) of these two groups of patients were compared.
Results
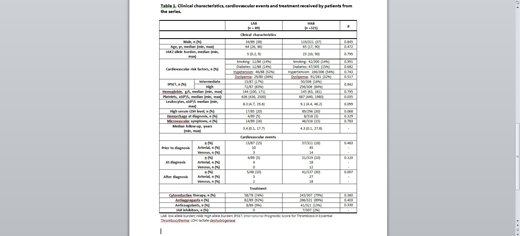
LAB and HAB groups did not significantly differ regarding the main clinical characteristics (i.e cardiovascular risk factors [CVRF] and International Prognostic Score for Thrombosis in Essential Thrombocythemia [IPSET] score) except for the median platelet count: LAB 636 x109/L [436- 2500] vs HAB 687 x109/L[440-1980L], p=0.035). CVE after diagnosis of ET were more frequent in patients with HAB (41/137, 30%) than in patients with LAB (5/48, 10%), p=0.007. Only one LAB patient with CVE had JAK2 allele burden >5%. Treatments received by both groups were not significantly different. None of the patients from both groups progressed to AML, whereas 1/48 vs. 6/137 of patients evolved to MF. Median follow-up of patients with LAB and HAB was 3.4 years [0.1-17.7] and 4.3 years [0.1-27.8], respectively (Table 1).
Conclusions
In these series of ET patients from the GEMFIN group, patients with LAB had significantly lower median platelet count at diagnosis and less CVE after diagnosis than patients with HAB, although CVRF and IPSET scores and treatment approach were similar. The clinical behavior of LAB patients may resemble that of individuals with CHIP. The therapeutic algorithm of ET patients with LAB may be somehow different than that of patients with HAB and therefore, might be revised.
Bellosillo:Astra-Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Biocartis: Honoraria; Merck-Serono: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Qiagen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Hoffman â€"La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; ThermoFisher: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; BMS: Honoraria. Hernandez Boluda:Incyte: Other: Travel expenses paid. Pérez:Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.