Introduction
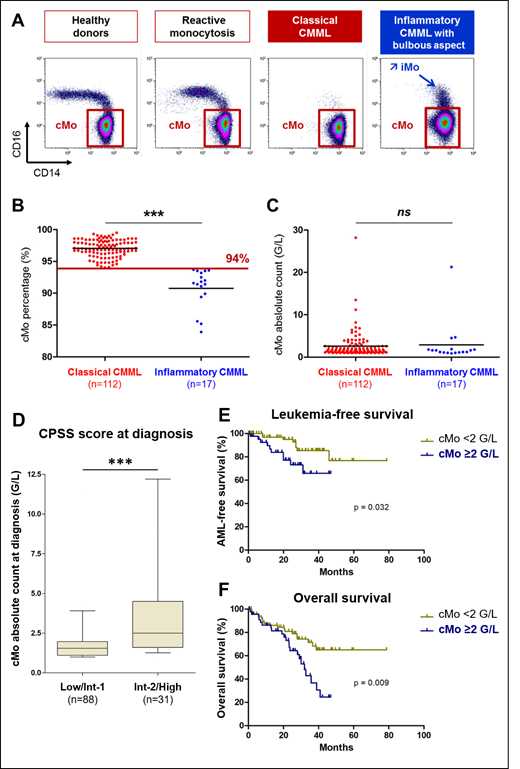
A relative increase in classical monocyte fraction (cMo, CD14++CD16-) over 94% of total peripheral blood monocytes, as measured by flow cytometry, distinguishes a CMML from a reactive monocytosis with a 94.1% specificity and a 92.8% sensitivity (Selimoglu-Buet, 2015; Talati, 2017; Patnaik, 2017; Selimoglu-Buet, 2017; Hudson, 2018). This phenotype is independent of the absolute monocyte count, WHO subtype of CMML, its dysplastic (MD) vs proliferative (MP) feature and its mutational background. The limitation of this diagnostic tool is the changes in monocyte subset repartition induced by an associated inflammatory disease that increase the fraction of intermediate monocytes (iMo, CD14++CD16+), leading to underestimation of cMo accumulation (Selimoglu-Buet, 2017). The present study explores the prognostic significance of cMo accumulation in CMML.
Patients and methods
Among CMML patients (diagnosis according to 2016 WHO criteria) included in our previous studies, we selected patients diagnosed from June 2012 to March 2017 in four centers in which follow-up was actualized in June 2019. Disease was classified according to WHO 2016 (CMML-0, -1, -2 and MP vs MD subtypes). When possible, the CMML Prognostic Scoring System (CPSS) was calculated. Peripheral blood flow cytometry data were reanalyzed in a blind fashion by a skilled operator. Inflammatory CMML were defined by a cMo percentage <94% associated with a bulbous aspect on flow cytometry profile (Figure 1A). The absolute cMo count in the peripheral blood was determined by combining flow cytometry measurement of cMo fraction and the total monocyte count provided by Complete Blood Count.
Results
One hundred and twenty-nine CMML patients (mean age: 75±10 years, sex ratio: 2.2) were classified into 53 CMML-0 (41%), 58 CMML-1 (45%) and 18 CMML-2 (14%) with 97 dysplastic forms (75%). Eight patients (6%) having received DNA damaging therapies (radiations or chemotherapies) were considered as secondary CMML. Eleven patients (9%) had evolved from a previous myelodysplastic syndrome. The median white blood count, hemoglobin level and platelet count were 9 G/l (IQR: 5.9-12.9), 11.8 g/dl (IQR: 9.7-13.1), and 121 G/l (IQR: 73-212), respectively. The CPSS score, calculated in 119 cases, classified the patients into "Low" (n=55), "Intermediate-1" (n=33), "Intermediate-2 (n=29) and "High" (n=2) categories. Twenty of the 114 patients (18%) with available follow-up (median duration: 26.7 months [13.9-36.5]) had received hypomethylating agents and 3 (3%) were allografted.
A cMo percentage above the threshold (97.0% [IQR: 96.3-98.1]) was detected in 112 patients (87%) whereas 17 (13%), referred thereafter as "inflammatory CMML", displayed cMo<94% (91.6% [IQR: 89.9-93.2], p<0.001) with an inflammatory state (C-reactive protein: 8 mg/L [IQR: 3.1-20.2] (Figure 1B). Importantly, the absolute count of cMo was similar in inflammatory (1.6 G/l [IQR: 1.1- 1.8]) and classical (1.7 G/l [IQR: 1.2- 2.6], p=0.25) CMML (Figure 1C). Focusing on total monocyte and cMo absolute counts, we did not detect any significant difference between CMML-0, CMML-1 and CMML-2. While cMo percentages were similar in MP and MD subtypes, the total monocyte (6.0 ±6.0 G/l vs 1.7 ±0.7 G/L, p<0.001) and the cMo (5.8 ±5.7 G/L vs 1.6 ±0.7 G/L, p<0.001) counts were increased in MP compared to MD CMML. A poor prognosis CPSS score at diagnosis also correlated with an increase in the absolute cMo count ("Intermediate-2 or High": 4.5 ±6.0 G/L vs "Low or Intermediate-1": 2.0 ±1.7 G/L; p <0.001) (Figure 1D).
A Receiver Operator Curve (ROC) defined a cut-off value of 2.0 G/L to identify patients with poorest outcome. Indeed, CMML patients with an absolute cMO count ≥2.0 G/L at diagnosis had a higher risk to progress to acute myeloid leukemia (AML) (HR=0.34 [95% CI, 0.12 - 0.91]) (Figure 1E). Eventually, patients with an absolute cMO count ≥2.0 G/L at diagnosis had poorer overall survival (OS) compared with patients with a lower absolute cMO count (HR=0.42 [95% CI, 0.22 - 0.80]; median OS: 31.9 months vs not reached, p=0.009) (Figure 1F).
Conclusion
These results demonstrate that in CMML patients, regardless of the relative cMo percentage, a high absolute count of circulating cMo at diagnosis ≥2.0 G/L correlates with a poor outcome, including a higher risk of AML progression and shorter survival.
Fenaux:Jazz: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Aprea: Research Funding. Wagner Ballon:Alexion: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract