OBJECTIVE: Pre-transplant chemotherapy can control progression of primary disease, alleviate disease and improve disease-free survival after transplantation in juvenile granulocyte leukemia (JMML).Methylation abnormalities play an important role in the development of JMML. This study was to explore the effectiveness of induction regimen including decitabine ,cytarabine and fludarabine before transplantation and to explore the affection to transplantation in JMML.
METHODS: A retrospective analysis of the remission and survival of 33 children with JMML before and after stem cell transplantation in the Department of Pediatrics of Nanfang Hospital from 2014.2 to 2019.7. There were fourteen girls and 19 boys, median diagnosis age 23 months(2m-10 years old). Median white blood cell count,median hemoglobin level,median platelet count( WBC) was 29.3G/L (6.29-158.66G/L); 83g /L(41-113g/L,); 27.17G / L(4-431G / L) respectively. Median Hemoglobin F level was 34.16%( 1.56-78%). Median spleen level under the costal margin was 5cm(0-13.3cm); Median liver under the costal margin was 3.9 cm(0 -8.9 cm) .There were 26 cases with the pulmonary involvement (26/33, 78.8%). The original blast cells in bone marrow were 0-8.8%, with a median of 4.5%.Mutant genes including: 2 cases of KAS, 9 cases of NF1, 2 cases of NRAS, 16 cases of PTPN11, and 4 cases without common JMML gene .The first course of treatment after diagnosis is 20 mg/m2 × 5 days of decitabine. The second course of treatment is DA: decitabine 20 mg/m2 × 5 days + cytarabine 100 mg/m2 × 5 days or A-3V regimen: Ara-C 100 mg/m2/d CIV×7 days+Etoposide 100 mg/m2/d ×5 days+Vincristine 1.5 mg/m2/d ×1 day. The third course of treatment is decitabine 20 mg/m2 × 5 days,then FLAG regimen: Fludarabine 30mg / m2 × 5 days, Ara-C 1 g / m2 × 5 days, G-CSF 5μg / Kg × 6 days. Single drug of decitabine 20 mg/m2 × 5 days was adminstered 1-3 times per monthly during the period of waiting for transplantation. Comprehensive assessment was performed before transplantation. Survival outcomes were analyzed by Kaplan-Meier curves.
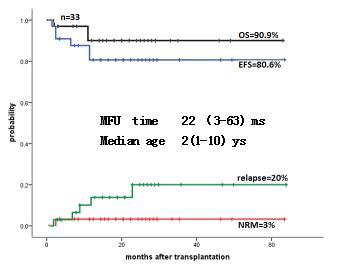
RESULTS: At least 3 courses of chemotherapy were completed in 33 cases, including 1-5 courses of decitabine.The bone marrow was evaluated in 31 patients before transplantation: 12 patients got complete remission(CR) and 19 patients got partial remission(PR) .Peripheral blood evaluation: 23 cases achieved WBC CR; 17 cases achieved platelet CR, 6 cases achieved platelet PR, 7 cases had no improvement. Eighteen cases of spleen were evaluated, of which only 3 cases were CR, 13 cases were PR, and 2 cases did not improve. Overall assessment, only 1 case achieved CR before transplantation, 1 case did not improve, and 31 cases achieved PR .Allogeneic hematopoietic stem cell transplantation was performed in 33 cases, including 3 cases of nonrelated peripheral blood hematopoietic stem cell transplantation(PB HSCT), 30 cases of complementary transplantation (haploid identical PB HSCT plus non-related cord blood transplantation). The median follow-up time after transplantation was 22m(3-63m).There was no death in the period of chemotherapy before transplantation. Four patients relapsed after transplantation, and One patient died of transplantation related death. Three years EFS was 80.6%.
Conclusion: Pre-transplant chemotherapy based on regimen of decitabine + cytarabine + fludarabine is safe, effective and feasible. The response rate of treatment is 97.0%, although the complete remission rate before transplantation is low, most of them are partial remission, but the disease-free survival after non-related HSCT or complementary transplantation could reached to 80.6%. These results indicated that pre-transplant chemotherapy based on regimen of decitabine + cytarabine + fludarabine was benefit to improved the survival of children with JMML after HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.