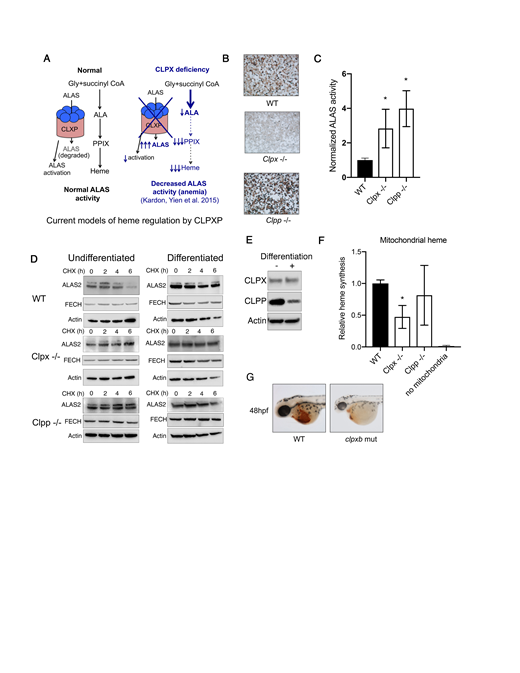
Differentiating erythroid cells synthesize large quantities of heme for hemoglobinization. While the transcriptional regulation and enzymatic mechanisms of the heme synthetic enzymes are well characterized, we lack mechanistic understanding of how their protein stability, cofactor incorporation and functional interactions with mitochondrial housekeeping proteins are regulated. These mechanisms can rapidly alter the rate of heme synthesis in response to external stimuli and metabolic requirements, and are critical for heme regulation within a tissue-specific and developmental context. CLPX, a mitochondrial protein unfoldase best understood for its function in a proteasome-like enzyme complex with the peptidase CLPP (the CLPXP ATP-dependent protease) plays a central role in regulation of mitochondrial protein turnover, is one such heme regulatory protein. CLPX activates yeast ALAS, which catalyzes the committed step of the heme synthesis pathway, by facilitating the incorporation of its cofactor, PLP, and is required for erythroid heme synthesis in zebrafish (Kardon et al. Cell 2015). Paradoxically, it regulates the turnover of ALAS1 and ALAS2 protein in vertebrate cell lines and appears to regulate the heme synthesis downstream of ALAS (Kubota et al. JBC 2016, Yien et al. PNAS 2017). However, it is not known if vertebrate ALAS was activated by ALAS, or if the requirement for CLPX in vertebrate heme synthesis was caused its regulation of ALAS activity (Figure A).
To dissect the roles of CLPX in erythroid heme synthesis, we knocked out Clpxand Clpp in murine erythroleukemia (MEL) cells and assayed the activity, stability, and steady state levels of the heme synthesis enzymes, ALAS2 and FECH, which colocalize with ALAS in the mitochondrial matrix. Consistent with previous observations, Clpx -/- MEL cells had a heme defect, while Clpp -/-cells did not (Figure B). However, in contrast to previous observations in the yeast model, CLPX is not required for ALAS activation in erythroid cells, but plays a key role in regulating ALAS2 turnover in concert with the CLPP peptidase (Figure C). During erythroid differentiation, CLPP protein levels are decreased, stabilizing ALAS2 protein (Figure D). Although differentiating Clpx -/-and Clpp -/- MEL cells did not demonstrate any changes in ALAS2 turnover, likely because steady-state levels of CLPP protein were already decreased (Figure E), we observed an increase in steady-state ALAS2 protein levels and a dramatic increase in ALAS2 enzyme activity. In vitro mitochondrial iron transport/heme synthesis assays revealed a heme defect in Clpx -/-MEL cells, suggesting that CLPX plays a role in mitochondrial iron metabolism. Collectively, these data suggest a complex, differentiation-stage specific regulation of heme synthesis by the CLPXP proteolytic complex (Figure F).
As Clpx -/- mouse embryos die by about E9.5 (mousephenotype.org), we dissected the in vivo role of Clpxin erythropoiesis by analyzing the phenotypes of clpxa and clpxb mutant zebrafish obtained from ZIRC. To accomplish this, we crossed clpxa and clpxb mutant zebrafish into Tg(lcr:GFP) zebrafish line in which erythroid cells are fluorescently labeled with GFP (Ganis et al Dev Biol 2012). We observed that clpxa mutant zebrafish had an early erythropoietic defect at 24 hpf that resolved at 48hpf; this developmental defect was not observed in clpxbmutant zebrafish. Benzidine staining of heme in mutant zebrafish revealed that while clpxa was dispensable for erythroid heme synthesis, clpxb was required for erythroid hemoglobinization (Figure G). Lastly, clpxbzebrafish mutants continued to be developmentally delayed and did not survive past 5 dpf. Collectively, our observations in cell lines and in the zebrafish model demonstrate that Clpx is essential for the maintenance of differentiated erythroid cells, as well as for the differentiation of the erythroid lineage.
The control of heme synthesis and erythroid development by CLPX reveals how mitochondrial physiology and heme synthesis are interdependent. Our results reveal an important regulatory node where the mitochondrial protein quality control machinery intersects with key steps in heme synthesis. Further, our studies provide important genetic tools for dissecting these regulatory components in isolation as well as within the in vivocontext of erythropoiesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.