Background: CMML is a heterogeneous disease with overlapping features of MDS and MPN. Several risk scores have been proposed in CMML, including CPSS (Such Blood 2013) that identifies 4 risk groups (low, intermediate-1 [int-1], int-2 and high risk) with median overall survival (OS) ranging from 12 months to > 5 years. HSCT is the only curative treatment in CMML, with an expected 20-40% post-transplantation OS. Current guidelines, common to MDS and CMML recommend rapidly transplanting higher-risk CMML and delaying transplant in lower-risk disease (de Witte Blood 2017). Retrospective transplant studies have shown better outcome in lower-risk CMML (Liu et al BBMT 2017). Whether this translates into a survival benefit compared to a delayed transplant strategy requires prospective studies. Multistate models based on retrospective data from transplant and non-transplant patients (pts) have addressed similar issues in MDS (Koreth JCO 2013, Della Porta Leukemia 2017). Through a large collaborative study, we address the question of optimal timing for HSCT in CMML patients through a similar large international collaborative study.
Method: We retrospectively selected pts from 2 registries: International MDS/MPN Working Group (IWG cohort, Padron Blood Cancer J 2015) and EBMT with the following criteria: WHO-defined CMML, age ≤ 70y, ECOG 0-2, diagnosis after 2000, available CPSS at the time of diagnosis.
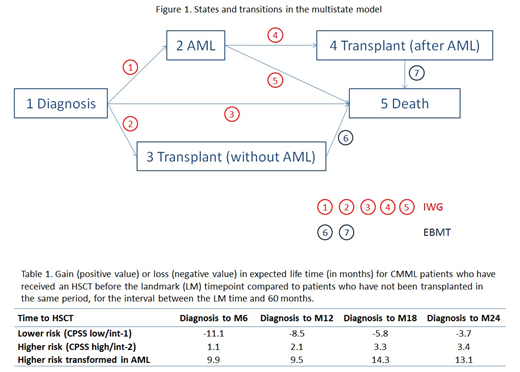
Different states were considered in higher- (CPSS int-2 and high) and lower- risk (CPSS low/int-1) pts: diagnosis, AML transformation, transplantation and death. IWG data was used to estimate transition probabilities from diagnosis to AML, transplantation and death and from AML to transplantation and death. EBMT data was used for transplantation to death transitions (Figure 1).
Results: 719 and 403 pts were identified in the IWG and EMBT registry, respectively (resp). Median age was 64 (range 16-70) in IWG and 58 (19-70) years in EBMT cohort. Patients were male in 69% and 67% of IWG and EBMT cohorts, respectively (resp). CPSS was low in 22% and 13%, int-1 in 31% and 31%, int-2 in 40% and 45% and high in 8% and 11% of the IWG and EBMT pts, resp. In the IWG cohort, the 1 year cumulative incidence of transformation into AML were 7.3% and 18.2% in lower and higher risk patients, resp. Among the 719 pts from IWG, 102 received HSCT. At the time of diagnosis the expected life time of higher risk (int-2 and high) patients was 25.2 months while it was 44.1 months in lower risk (int-1 and low) patients.
Table 1 reports gain or loss in expected life time (until 60 months after diagnosis) for transplantation at different intervals from diagnosis (within 6, 12, 18 and 24 months) for patients who have survived and not transformed to AML until the prediction time point, taking into account CPSS at diagnosis. Expected life time is also given separately for higher risk pts after transformation into AML. There was a modest gain of life expectancy with transplantation in higher risk patients (CPSS int-2 and high) while it was not the case in lower risk patients. The survival gain in higher risk pts with HSCT rose from 1.11 at 6 months to 3.4 months at 24 months. There was also a survival benefit of HSCT in higher risk pts who were transformed to AML during their follow-up which was even higher in terms of gain of months: from 9.89 month if HSCT performed within 6 months to 14.25 months if performed within 18 months.
Of note, the loss of survival in lower risk patients who underwent HSCT decreased over time: 11.1 months if HSCT is performed within 6 months and 3.7 months if HSCT is performed within 24 months, probably owing to transition to higher-risk CMML at this stage in some pts, a transition that could not be captured in the present dataset.
Conclusion: For the first time, using a multistate model, we could provide evidence that the transplant benefit in CMML is restricted to higher-risk pts, in line with findings in MDS and current guidelines. More analyses will be performed to analyze other subgroups of patients, especially regarding their risk (other classification than CPSS, the role of somatic mutation…). More studies are needed to analyze the role of pre HSCT treatment and its potential impact on post-transplant outcome.
Robin:Novartis Neovii: Research Funding. Beelen:Medac GmbH Wedel Germany: Consultancy, Honoraria. Fenaux:Celgene Corporation: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Aprea: Research Funding. Kroeger:Riemser: Research Funding; Novartis: Honoraria, Research Funding; Medac: Honoraria; DKMS: Research Funding; Neovii: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; JAZZ: Honoraria; Sanofi-Aventis: Honoraria. Nazha:Tolero, Karyopharma: Honoraria; MEI: Other: Data monitoring Committee; Novartis: Speakers Bureau; Jazz Pharmacutical: Research Funding; Incyte: Speakers Bureau; Daiichi Sankyo: Consultancy; Abbvie: Consultancy. Rampal:Agios, Apexx, Blueprint Medicines, Celgene, Constellation, and Jazz: Consultancy; Constellation, Incyte, and Stemline Therapeutics: Research Funding. Finke:Riemser: Honoraria, Other: research support, Speakers Bureau; Neovii: Honoraria, Other: research support, Speakers Bureau; Medac: Honoraria, Other: research support, Speakers Bureau. Komrokji:Agios: Consultancy; DSI: Consultancy; pfizer: Consultancy; celgene: Consultancy; JAZZ: Consultancy; Novartis: Speakers Bureau; JAZZ: Speakers Bureau; Incyte: Consultancy. Killick:JAZZ: Honoraria; Celgene: Honoraria; NOVARTIS: Honoraria, Membership on an entity's Board of Directors or advisory committees; ALEXION: Honoraria. Blaise:Molmed: Consultancy, Honoraria; Sanofi: Honoraria; Pierre Fabre medicaments: Honoraria; Jazz Pharmaceuticals: Honoraria. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Patnaik:Stem Line Pharmaceuticals.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract