Introduction. Allogeneic stem cell transplantation (SCT) can be curative for those with high-risk or recurrent hematologic cancers including T-cell non-Hodgkin's lymphoma (TCL). For patients lacking an HLA-matched related donor, donor sources such as haplo-identical (Haplo) relatives or cord blood (UCB) represent reasonable alternatives. There is currently little data on the outcome of TCL patients undergoing alternative donor transplantation. The Société Francophone de Greffe de Moelle et de Transplantation Cellulaire (SFGM-TC) conducted this study to evaluate the outcome of recipients of alternate donor allo-SCT for TCL.
Methods. 95 patients with non epidermotropic TCL, transplanted between 2003 and 2017, reported to ProMISE (Project Manager Internet Server), an internet-based system shared by 36 Francophone transplantation centers, were reviewed. Comparisons of outcome after transplants from different donors (Haplo, n=41 vs UCB, n=54) were performed with regard to overall survival (OS), non-relapse mortality (NRM), relapse and acute/chronic graft-versus-host disease (aGVHD/cGVHD) incidence.
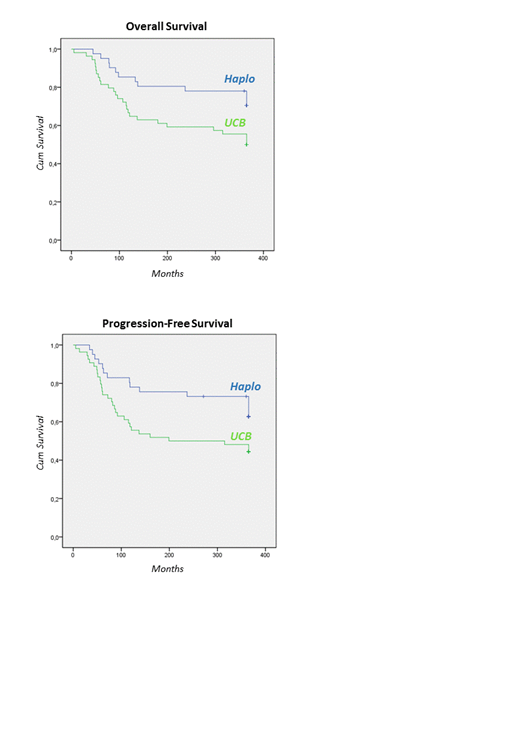
Results. Patients' characteristics are detailed in Table 1. Median age at HSCT was 42.5 (range, 3.6 - 68) years, with an older cohort for the Haplo group (50.7 vs 33.6 for UCB, p< .001). Except for the median year of transplantation (median year 2017 for Haplo vs 2010 for UCB,) and the myeloablative regimen (22% for Haplo vs 48% for UCB, p .009), both Haplo and UCB groups were comparable concerning interval from diagnosis to HSCT, donor/recipient CMV status, sex and ABO mismatch. Patients received a median of 2 (range, 1-5 for Haplo; 1-6 for UCB) therapeutic lines. Twenty-eight (10 in Haplo vs 18 in UCB, p NS) underwent auto- or allo-SCT prior to Haplo or UCB-HSCT. Median time to engraftment was shorter (19 vs 25 days, p .025) in Haplo recipients. According to donor type, no differences were found in univariate analysis for the CI of grade II-IV aGVHD (35% vs 35%, p .88) and cGVHD (30% vs 28%, p .49). Median follow-up was 24 months and 47 months for Haplo and UCB recipients, respectively (p .004). At 12 months, OS was 71% and 50% (p .033), progression-free survival (PFS) 63% and 44% (p .045) and NMR 7% and 26% (p .014) in HSCT from Haplo and UCB, respectively. Relapse incidence was similar following HSCT from Haplo and UCB (32% vs 31%).
Conclusion. Based on these first results, our study suggests that Haplo-HSCT is a strategy achieving prolonged survival as compared to UCB donors, due to less toxicity and similar incidence of relapse. An analysis of influencing factors will be further detailed.
Blaise:Jazz Pharmaceuticals: Honoraria; Pierre Fabre medicaments: Honoraria; Molmed: Consultancy, Honoraria; Sanofi: Honoraria. Socie:Alexion: Consultancy. Mohty:Jazz Pharmaceuticals: Honoraria, Research Funding. Robin:Novartis Neovii: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.